Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: To report the demographics, clinical characteristics, and differential impacts on quality of life measures among participants in the Psoriasis (PsO) and Psoriatic Arthritis (PsA) Registry (COPPAR).
Methods: The COPPAR registry is a prospective, longitudinal registry for patients ≥ 18 years of age who have a diagnosis of PsO or PsA and were enrolled from a dermatology-rheumatology combined clinic and an arthritis center at a large academic medical center. Descriptive characteristics and biospecimens were collected every six months. Patient data included demographics, clinical and patient-reported outcomes, comorbidities, and treatment history. Baseline demographics and disease characteristics at enrollment were summarized for each group and compared using univariate statistics. Quality of life outcomes were compared between PsO and PsA subjects in regression analyses adjusting for age and sex.
Results: As of April 30, 2021, 154 subjects were enrolled, including 28 PsO subjects and 126 PsA subjects. Demographic characteristics were mean age of 53.2 years, 55.2% female, 89% white, and 25.3% were overweight and 35.1% were obese (Table 1). The mean disease duration was 19.7 years [SD 11.1] for PsO and 13.3 years [SD 14.4] for PsA (p< .001) (Table 2). PsO subjects had a higher mean BSA compared with PsA subjects (4.4 % [SD 5.4] vs. 3.2% [SD 8.7], p< .001). Both PsO and PsA groups had a high percentage of biologic therapy (60.7 % for PsO and 78.5 % for PsA). The mean static physician's global assessment (sPGA) score was higher among PsO subjects (2.1 [SD 1.0] compared to PsA subjects (1.2 [SD 1.1], p< 0.001). The mean Psoriasis Area and Severity Index (PASI) was higher for PsO subjects (4.5 [SD 5.6] for PsO vs. 2.3 [SD 4.7] for PsA subjects(p< .001)). Multivariate comparisons of quality of life measures between PsO and PsA subjects indicated lower EuroQol- 5 Dimension (EQ-5D) scores in PsA subjects (PsA: mean 0.8 [SD 0.1] vs. PsO: mean 0.9 [SD 0.1], p< .001) and lower physical health status (SF-12 PCS) among PsA subjects (mean 43.7 [SD 11.6]) compared with PsO subjects (mean 49.2 [SD 11.3], p=0.04) (Table 3). There were no significant differences between the groups in PHQ-8, PROMIS anxiety, FACIT-fatigue, or Dermatology Life Quality Index (DLQI) scores. PsA subjects had slightly more daily work activity impairment (WPAI PsA: mean 16.7% [SD 22.8] vs. PsO: mean 6.0% [SD 14.5], p=0.08). There were no significant differences in patient global skin scores between PsO subjects (mean 7.4 [SD 2.1]) and PsA subjects (mean 5.9 [SD 5.7], p=0.4).
Conclusion: Subjects enrolled in a combined dermatology-rheumatology and arthritis center clinic at a tertiary academic medical center had mild to moderate disease activity with a high prevalence of biologic therapy. PsA subjects reported worse general physical health and lower quality of life than PsO subjects despite PsO subjects having worse overall psoriasis severity.
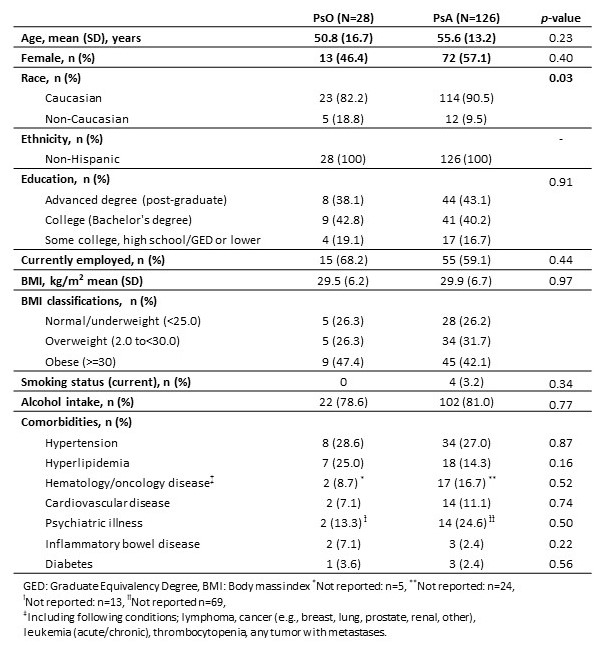 Table 1. PsO and PsA Patient Characteristics
Table 1. PsO and PsA Patient Characteristics
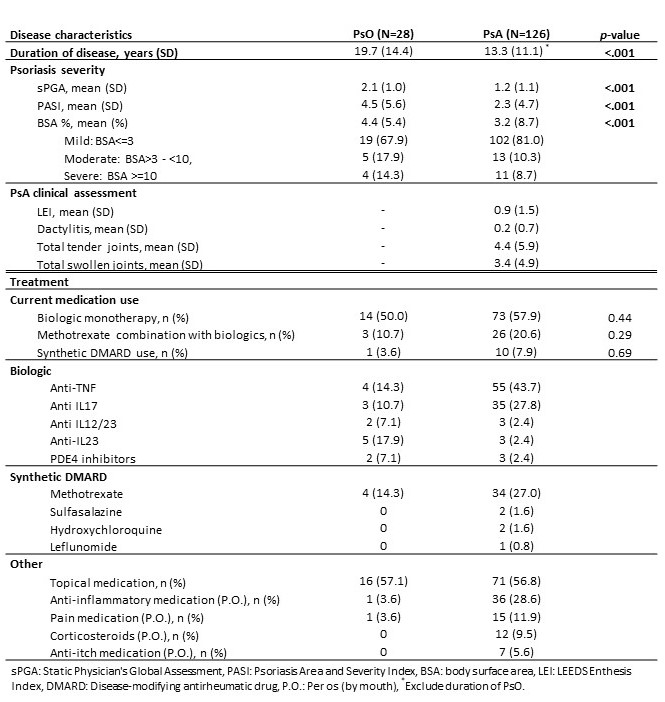 Table 2: PsO and PsA Disease Characteristics and Treatment
Table 2: PsO and PsA Disease Characteristics and Treatment
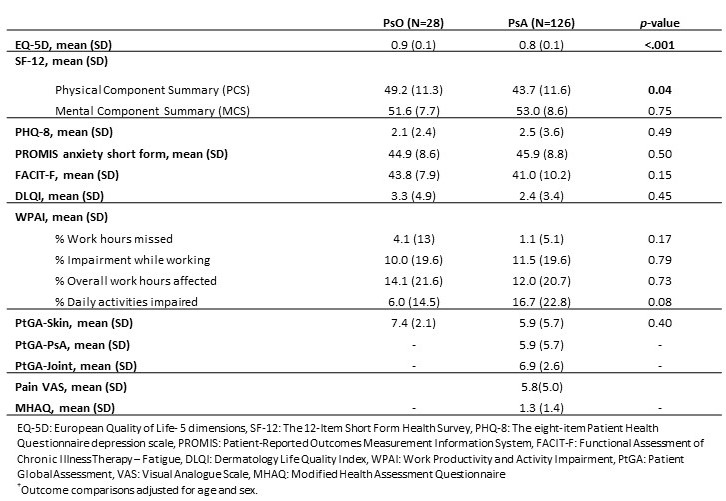 Table 3: Multivariate Comparisons of Quality of Life Outcome Measures between PsO and PsA subjects+
Table 3: Multivariate Comparisons of Quality of Life Outcome Measures between PsO and PsA subjects+
To cite this abstract in AMA style:
Shadick N, Schnock K, Feather V, Cui J, Maica G, Marshall A, Francis W, Yussuff M, Perez Chada L, Weinblatt M, Merola J. A Comparison of Quality of Life Outcomes Between Psoriatic Arthritis and Psoriasis Patients: Data from the Brigham Cohort for Psoriasis and Psoriatic Arthritis Registry (COPPAR) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/a-comparison-of-quality-of-life-outcomes-between-psoriatic-arthritis-and-psoriasis-patients-data-from-the-brigham-cohort-for-psoriasis-and-psoriatic-arthritis-registry-coppar/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-comparison-of-quality-of-life-outcomes-between-psoriatic-arthritis-and-psoriasis-patients-data-from-the-brigham-cohort-for-psoriasis-and-psoriatic-arthritis-registry-coppar/
