Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Upadacitinib (UPA), a selective JAK1 inhibitor, has demonstrated efficacy and safety in patients with rheumatoid arthritis (RA) as monotherapy and in combination with conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) such as methotrexate (MTX).1,2 However, UPA monotherapy has not been compared directly with UPA combination therapy in the Phase 3 program.
We compare the efficacy of UPA monotherapy and UPA in combination with MTX using data from two Phase 3 trials of RA patients with an inadequate response (IR) to prior MTX therapy.
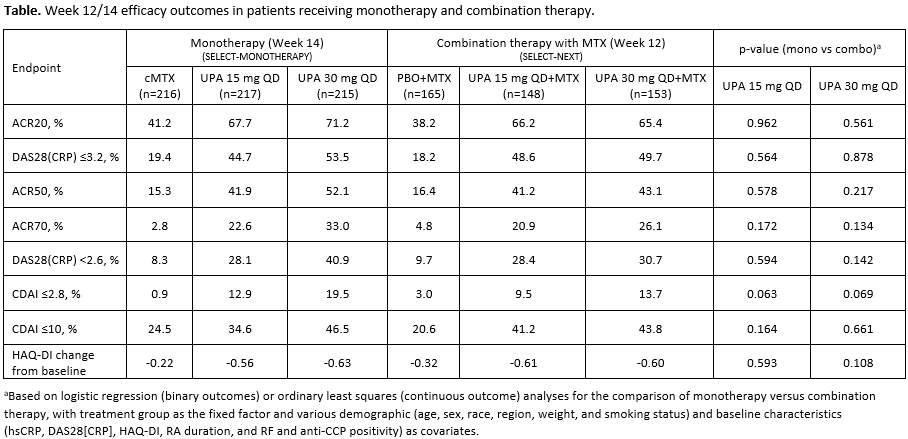
Methods: In SELECT-MONOTHERAPY, 648 MTX-IR patients were randomized to receive UPA 15 mg or 30 mg monotherapy once daily (QD), or continue with MTX monotherapy (cMTX; given as a blinded study drug), for 14 weeks. In SELECT-NEXT, 661 csDMARD-IR patients were randomized to receive UPA 15 mg or 30 mg QD or placebo (PBO) for 12 weeks on a background of csDMARDs. Only patients receiving concomitant MTX (with or without additional csDMARDs) at baseline in SELECT-NEXT were included in this analysis. The primary endpoints of both studies were the proportion of patients achieving ACR20 and DAS28(CRP) ≤3.2. Additional endpoints included ACR50/70, DAS28(CRP) < 2.6, CDAI remission (≤2.8), CDAI low disease activity (LDA; ≤10), and change from baseline in HAQ-DI. Logistic regression or ordinary least squares analyses were used to compare outcomes with monotherapy versus combination therapy, adjusting for demographics and baseline disease characteristics. Results: A total of 1114 patients were included in the analysis, of whom 648 received monotherapy in SELECT-MONOTHERAPY and 466 received combination therapy in SELECT-NEXT. Of the patients receiving combination therapy, 338 (72.5%) were receiving MTX background therapy only and 128 (27.5%) were receiving MTX plus other csDMARDs. Baseline characteristics were generally similar between the study cohorts; the majority of patients in both studies were female and of white ethnicity, with a mean age of approximately 55 years and a mean MTX dose of approximately 17 mg/week. Consistent with previously reported results from SELECT-MONOTHERAPY1 and SELECT-NEXT,2 both UPA monotherapy and UPA combination therapy led to significant improvements in efficacy outcomes versus cMTX/PBO+MTX (Table). No significant differences were observed between UPA monotherapy and UPA combination therapy across a range of clinical endpoints, including ACR20/50/70 responses and measures of LDA and remission. In addition, improvements in quality of life as measured by HAQ-DI were similar with UPA monotherapy and combination therapy. Efficacy was comparable between the two UPA doses in the combination therapy group, whereas in the monotherapy group numerically higher responses were observed with UPA 30 mg versus UPA 15 mg.
Conclusion: In MTX-IR patients with RA, the efficacy of UPA appears comparable when administered as monotherapy or when given in combination with MTX.
1. Smolen J, et al. Ann Rheum Dis 2018;77:67─8
2. Burmester GR, et al. Lancet 2018;391:2503–12
To cite this abstract in AMA style:
Buch M, Wells A, Rubbert-Roth A, Jain M, Schlacher C, Camp H, Li Y, Song Y, Nash P. A Comparative Analysis of Upadacitinib Monotherapy and Upadacitinib Combination Therapy for the Treatment of Rheumatoid Arthritis from Two Phase 3 Trials [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-comparative-analysis-of-upadacitinib-monotherapy-and-upadacitinib-combination-therapy-for-the-treatment-of-rheumatoid-arthritis-from-two-phase-3-trials/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-comparative-analysis-of-upadacitinib-monotherapy-and-upadacitinib-combination-therapy-for-the-treatment-of-rheumatoid-arthritis-from-two-phase-3-trials/