Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Cryopyrin-associated periodic syndromes (CAPS) are monogenic autoinflammatory diseases with severe systemic inflammation. The IL-1β inhibitor canakinumab (CAN) leads to a rapid remission of CAPS symptoms in clinical trials as well as in practice. The RELIANCE registry investigates the long-term safety and efficacy of CAN under routine clinical conditions in pediatric (≥2 years) and adult patients with CAPS, including MWS, FCAS, and NOMID/CINCA (CINCA: chronic infantile neurologic cutaneous articular syndrome, FCAS: familial cold-induced autoinflammatory syndrome, MWS: muckle-wells syndrome, NOMID: neonatal multisystem inflammatory syndrome)
Methods: This prospective, non-interventional, observational study enrolls patients with a clinically confirmed diagnosis of CAPS who routinely receive CAN. Clinical data, physician assessments, and patient-reported outcomes will be collected at baseline and at 6-monthly visits.
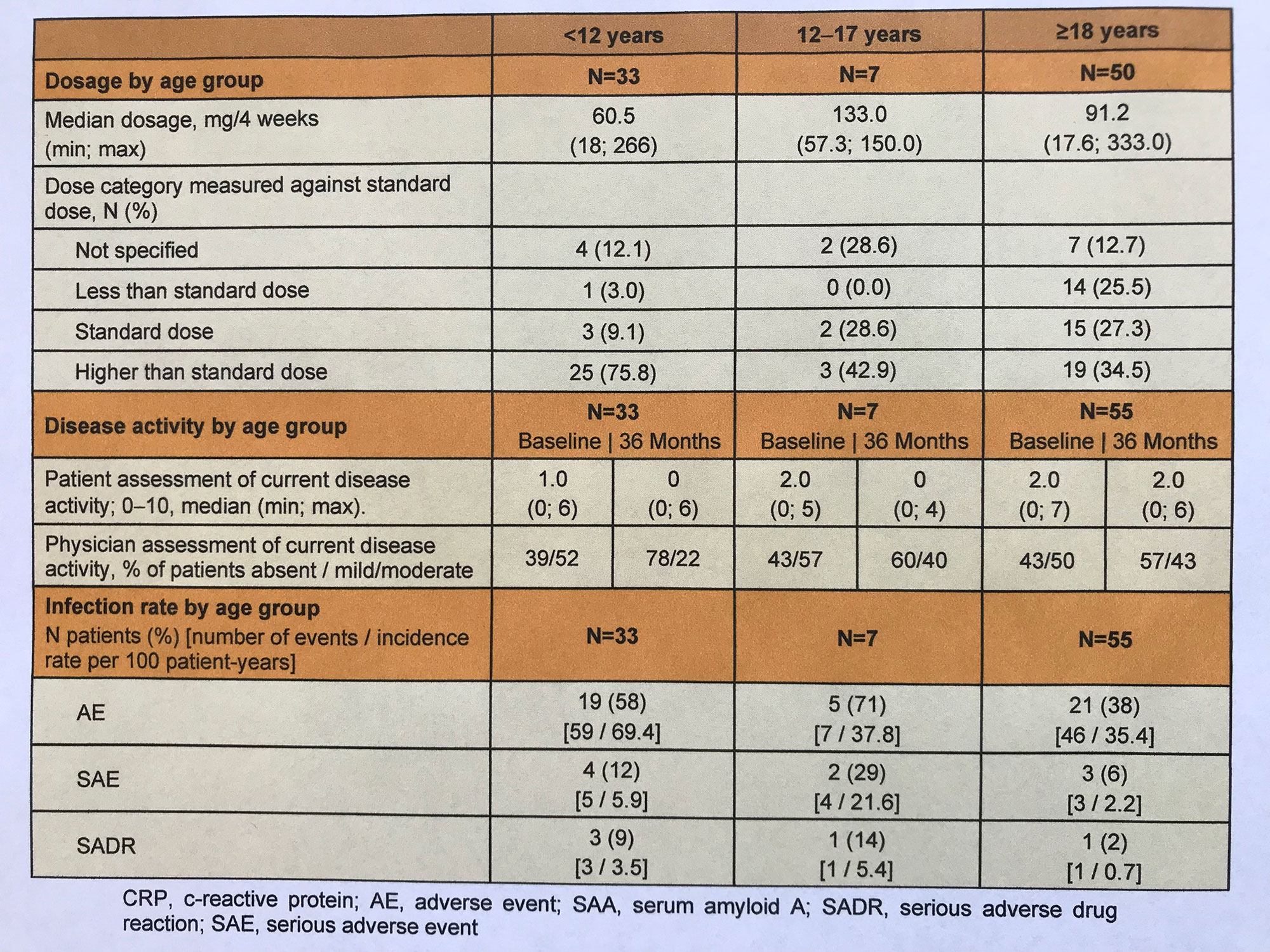
Results: 98 CAPS patients (52% female; median age 20 years; median duration of prior CAN treatment 6 years) were enrolled in the study through December 2021. At the 36-months visit, both physicians and patients of all ages rated current disease activity as absent or mild/moderate (Table 1). While one-third of adult patients each received less than the standard dose, the standard dose, or a higher dose, the proportion of pediatric CAPS patients with higher doses was significantly greater ( < 12 years: 76%, 12-17 years: 43%). The proportion of patients without disease activity was highest in < 12-year-old patients (78%). Proportionate, more AE, SAE, and presumably drug-related SAE occurred in the pediatric cohort. Pathogenic mutations were documented for a total of N=38 patients, including R260W: N=15, A439V: N=9, T348M: N=9, D303N: N=3, and E627G, G755R, and G569R: N=1 each. Disease activity in these patients according to physician rating was absent and mild/moderate at a ratio of 1:1 (N=18:20). In contrast, this ratio was 2:1 in all other patients (N=38:19). Severe disease activity occurred in one patient with the V198M mutation. N=27 patients with pathogenic mutation received standard dose CAN and N=9 patients received higher dose.
Conclusion: The 36-months interim analysis of the RELIANCE study shows that long-term treatment with CAN is safe and effective in patients with CAPS regardless of the underlying mutation. Pediatric patients tend to have a higher infection rate with a better response rate.
To cite this abstract in AMA style:
Kuemmerle-Deschner J, Kortus-Goetze B, Oommen P, Janda A, Rech J, Schuetz C, Kallinich T, Weller-Heinemann F, Horneff G, Foeldvari I, Meier F, Borte M, Krickau T, Weber-Arden J, Blank N. 3-years Safety and Efficacy Outcomes of Canakinumab Treatment in Cryopyrin-associated Periodic Syndromes (CAPS) – Data from the RELIANCE Registry [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/3-years-safety-and-efficacy-outcomes-of-canakinumab-treatment-in-cryopyrin-associated-periodic-syndromes-caps-data-from-the-reliance-registry/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/3-years-safety-and-efficacy-outcomes-of-canakinumab-treatment-in-cryopyrin-associated-periodic-syndromes-caps-data-from-the-reliance-registry/