Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Upadacitinib (UPA) was approved in 2020 in Japan for “the treatment of Rheumatoid arthritis (RA) with inadequate response to conventional therapy (including inhibition of the progression of structural damage)”. In Japanese label, UPA15 mg should be orally administered once daily for adult patients (pts). UPA 7.5 mg may be acceptable for some pts according to their condition. An interim analysis of all-case PMS was performed to evaluate the safety and effectiveness of RA pts with UPA in a clinical setting.
Methods: This PMS registered all RA pts treated with UPA in Japan, updating the interim analysis with additional cases since last year’s report.1
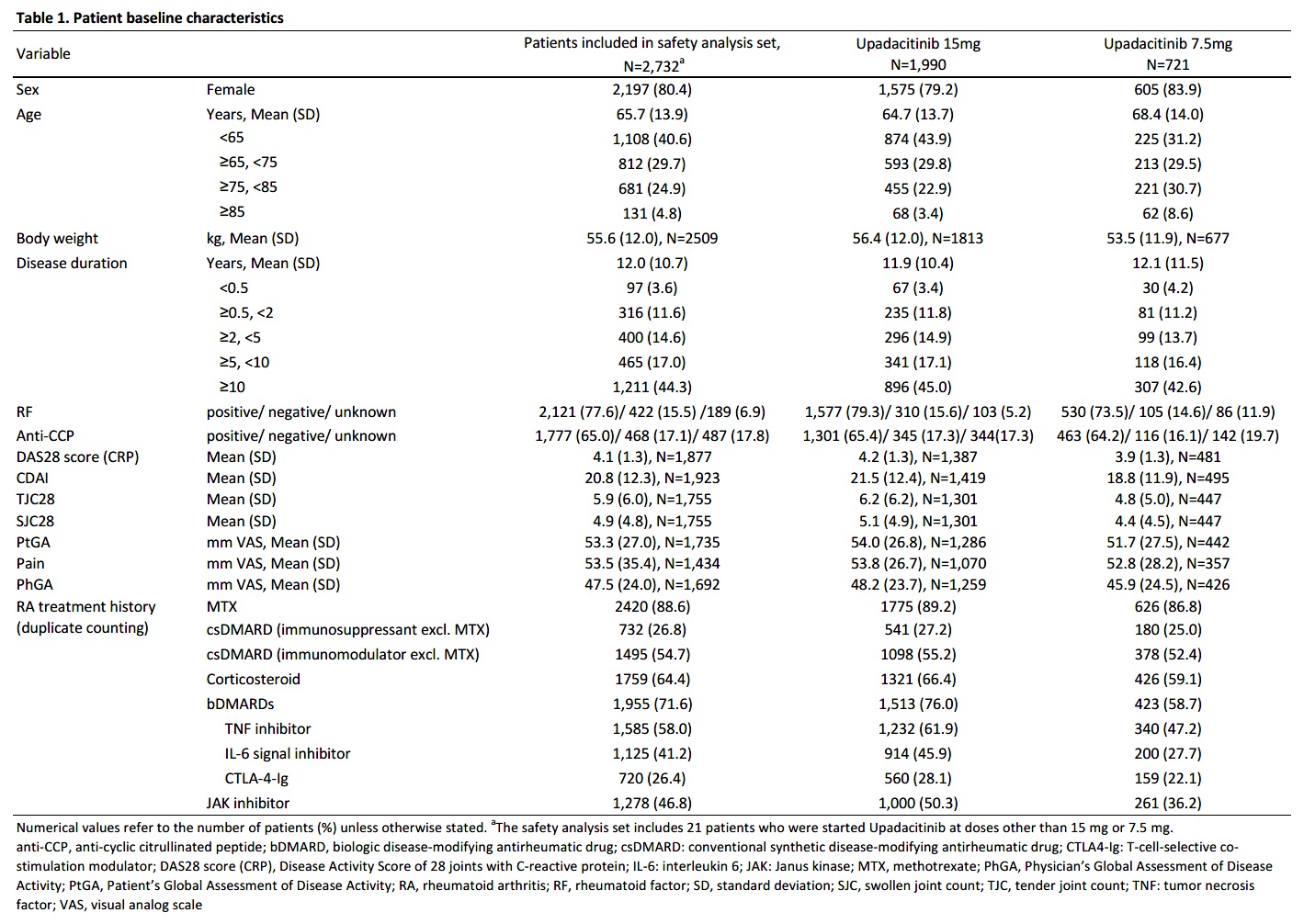
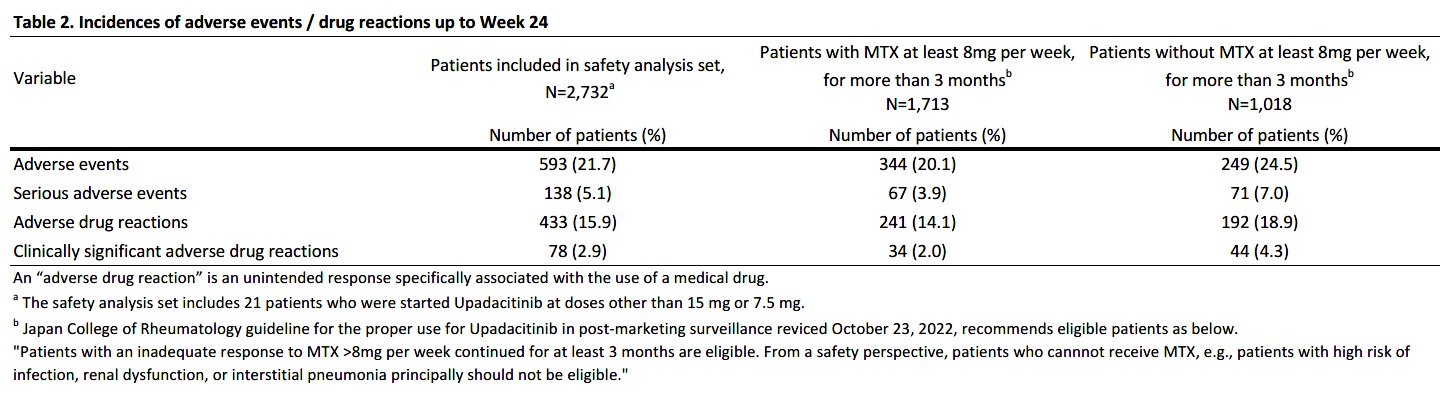
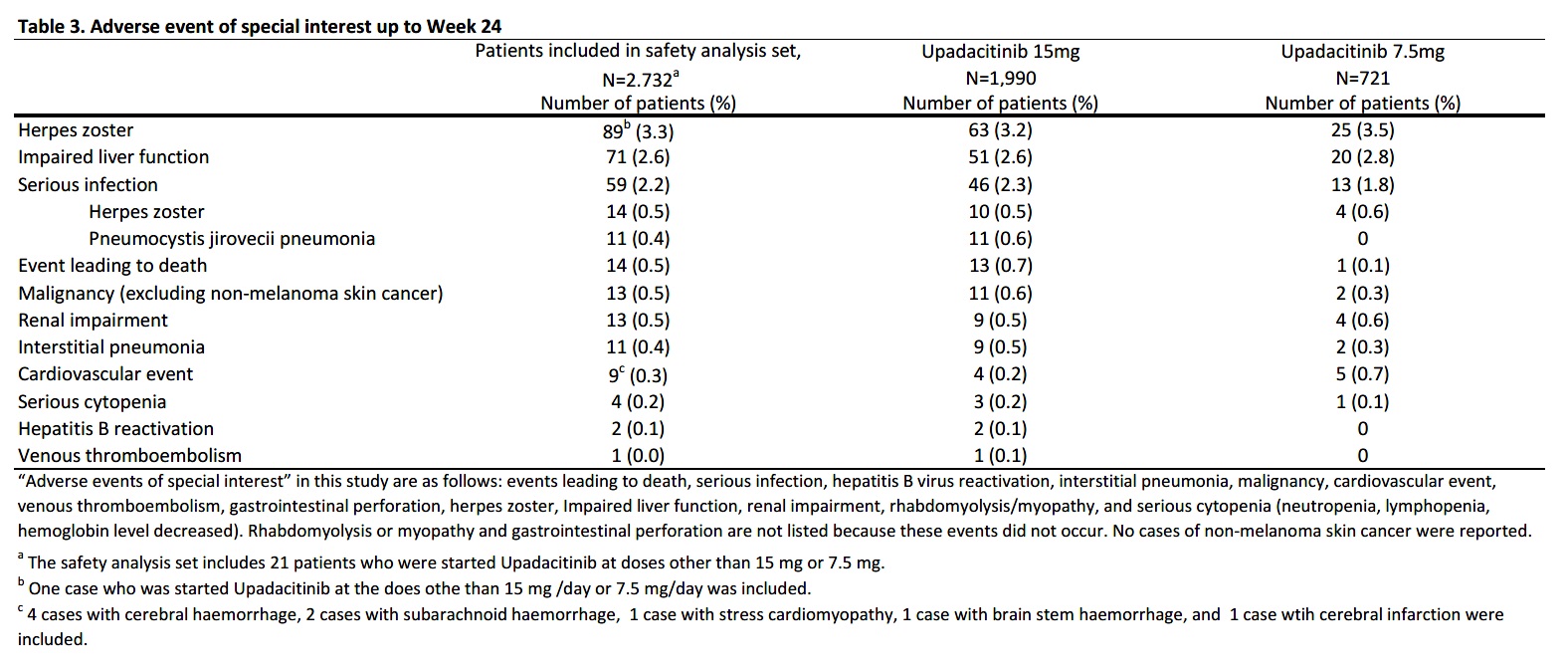
Results: As of Aug 15, 2023, 2,934 pts were enrolled, with 2,732 and 1,893 included in the safety and efficacy analysis sets, respectively. 79.8% (2,180 pts) of the safety analysis set continued UPA for 24 weeks. Initial doses were 15 mg/day (72.8%, n=1,990), 7.5 mg/day (26.4%, n=721), and others (0.8%, n=21). Mean ages were 64.7 (15 mg) and 68.4 years (7.5 mg), with mean RA durations of 11.9 and 12.1 years, respectively. History of treatment with biological DMARDs (bDMARDs) and JAK inhibitors (JAKi) was reported in 71.6% and 46.8%, respectively (Table 1). Adverse events (AEs) occurred in 593 (21.7%) and serious AEs (SAEs) in 138 (5.1%). SAEs included 10 cases of interstitial pneumonia and 11 of pneumocystis jirovecii pneumonia (PCP), each at 0.4 %. Among adhering to the JCR proper use guideline of UPA in PMS recommending MTX >8 mg/week for ≥3 months prior to initiating UPA,2 AEs in 344 of 1,713 pts (20.1%) and SAEs in 67 (3.9%). Comparatively, 1,018 non-compliant pts had higher rates: AEs in 249 (24.5%) and SAEs in 71 (7.0%) (Table 2). 14 deaths included one case in the 7.5 mg cohort (subarachnoid hemorrhage (SAH)), and 13 in the 15 mg cohort: three lung tumor, one recurrent pancreatic cancer, one SAH, one organizing pneumonia, one bacterial pneumonia, two PCP, one COVID-19, one malignancy neoplasm, and two unknown. AEs of special interest for each cohort include herpes zoster (3.2%, 3.5%), serious infections (2.3%, 1.8%), malignancy (0.6%, 0.3%), cardiovascular events (0.2%, 0.7%), interstitial pneumonia (0.5%, 0.3%), and venous thromboembolism (0.1%, 0) (Table 3). Among the 13 malignancy cases, seven were diagnosed within two months of starting UPA.
In the efficacy analysis set of 1,389 pts started on 15mg, 865 (62.3%) were assessed for DAS28-CRP at week 24: 701 (50.5%) reached DAS28-CRP< 3.2.
The study had limitations; 26.4% of pts were initiated on 7.5mg without documented reasons, and 37.3% did not meet the JCR proper use guideline of UPA in PMS, which recommends prior MTX >8 mg/week for ≥3 months.2
Conclusion: The observed safety and effectiveness profile was consistent with that reported in clinical trials and the previous report,1, 3, 4 with no new safety signals identified. AEs rates were similar across doses, indicating no definitive enhanced safety with lower 7.5mg dose. Compliance with the JCR proper use guideline and initiating 15 mg unless appropriate reasons are crucial in clinical settings in Japan.
References:
1 Mod Rheum suppl 2023(33): S179
2 JCR proper use guideline of UPA in PMS
3 Burmester GR, et al., RMD Open 2023; 9 (1): e002735
4 Yamaoka K, Drug Saf. 2021 Jun;44(6):711-722
To cite this abstract in AMA style:
Fujii T, Okamoto N, Abe A, Takagi M, Takahashi N, Nakajima A, Nakajima A, Nakayamada S, Nishida K, Kawaberi T, Sunaga N, Tsujita Y, Chonan S, Kuwana M, Tanaka Y. 24-week, Post-Marketing Surveillance Analysis of Upadacitinib in Japanese Patients with Rheumatoid Arthritis: The 2024 Interim Report [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/24-week-post-marketing-surveillance-analysis-of-upadacitinib-in-japanese-patients-with-rheumatoid-arthritis-the-2024-interim-report/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/24-week-post-marketing-surveillance-analysis-of-upadacitinib-in-japanese-patients-with-rheumatoid-arthritis-the-2024-interim-report/