Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose:
In 2010 the latest ASAS-EULAR recommendations for AS and the ASAS recommendations for the use of TNF-inhibitors (TNFi) have been published. Since then new treatments for axSpA have become available. We aimed to update and integrate the two sets of recommendations into one set applicable to patients with axSpA.Methods:
The EULAR Standardised Operating Procedures have been followed. First, two Systematic Literature Reviews have been performed to update the evidence on all treatment options (pharmacological and non-pharmacological) since 2009. The results have been presented during a one-day meeting of the task force. Thereafter, overarching principles and recommendations were updated by a process of achieving consensus and voting.Results:
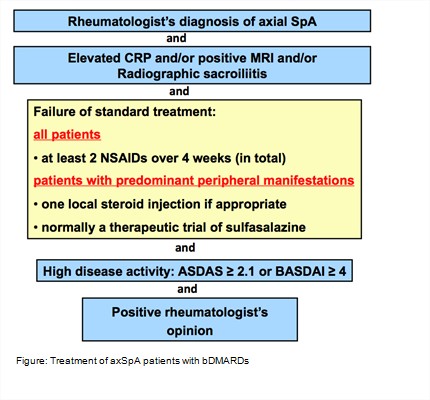
A total of 5 overarching principles and 13 recommendations have been formulated (Table). The first 3 recommendations deal with personalised medicine including treatment target and monitoring. Recommendation 4 deals with non-pharmacological management. Recommendation 5 describes the central role of NSAIDs as first pharmacological treatment. Recommendations 6 to 8 define the limited place of analgesics, glucocorticoids and conventional synthetic DMARDs. Biological DMARDs (bDMARDs) include TNF- and IL17-inhibitors and are indicated in patients diagnosed with axSpa by a rheumatologist, who have radiographic sacroiliitis and/or inflammation on MRI and/or an elevated CRP-level. Patients should also have high disease activity despite the use of -or intolerance for- at least 2 NSAIDs. High disease activity is defined as an ASDAS ≥2.1 or BASDAI ≥4 and an indication to start a bDMARD by a rheumatologist (Figure). The continuation of a bDMARD should be considered if an improvement of ASDAS ≥1.1 or BASDAI ≥2 has been achieved after at least 12 weeks. Current practice is to start with a TNFi. Switching to another TNFi or an IL-17i is recommended in case of failure of TNFi treatment. Tapering -but not stopping- of a bDMARD can be considered in patients with sustained remission. The final two recommendations deal with surgery and fractures.Conclusion:
The 2016 ASAS-EULAR recommendations provide up-to-date guidance on management of patients with axSpA.
Disclosure: D. van der Heijde, AbbVie, Amgen, Astellas, AstraZeneca, BMS, Celgene, Daiichi, Eli-Lilly, Galapagos, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis, UCB, 5,Director of Imaging Rheumatology bv, 3; S. Ramiro, None; R. Landewé, None; X. Baraliakos, AbbVie, BMS, Celgene, Chugai, Merck, Novartis, Pfizer, UCB, and Werfen, 2,AbbVie, BMS, Celgene, Chugai, Merck, Novartis, Pfizer, UCB, and Werfen, 5,AbbVie, BMS, Celgene, Chugai, Merck, Novartis, Pfizer, UCB, and Werfen, 8; F. van Den Bosch, AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Merck, Novartis, UCB Pharma, 5,AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Merck, Novartis, Pfizer, UCB Pharma, 8; A. Sepriano, None; A. Regel, None; J. D. Reveille, None; J. Braun, Abbott, Bristol-Myers Squibb, Celgene, Celltrion, Chugai, Johnson & Johnson, MSD, Novartis, Pfizer, Roche, UCB Pharma, 5,Abbott, Bristol-Myers Squibb, Celgene, Celltrion, Chugai, Johnson & Johnson, MSD, Novartis, Pfizer, Roche, UCB Pharma, 2.
To cite this abstract in AMA style:
van der Heijde D, Ramiro S, Landewé R, Baraliakos X, van Den Bosch F, Sepriano A, Regel A, Reveille JD, Braun J. 2016 Update of the ASAS-EULAR Management Recommendations for Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/2016-update-of-the-asas-eular-management-recommendations-for-axial-spondyloarthritis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/2016-update-of-the-asas-eular-management-recommendations-for-axial-spondyloarthritis/