Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Over 40% of patients with OA report having significant knee pain every day. (1) Previously published data have demonstrated the overall comparable efficacy of EC naproxen/IR esomeprozole to celecoxib and superiority to placebo in the management of knee OA. (2) EC naproxen/IR esomeprazole also significantly reduced the incidence of endoscopic ulcers and improved UGI tolerability compared with EC naproxen alone in previous trials and maintained GI protective effect with low-dose aspirin (3). This new analysis characterizes time-to-first pain relief, effect size, and sustainability of naproxen/IR esomeprazole and celecoxib with placebo.
Methods: Two double-blind, double-dummy, placebo-controlled trials in patients aged ≥50 years with knee OA randomized to either EC naproxen 500mg/IR esomeprazole 20mg BID (n=487) or celecoxib 200mg/day (n=486) or placebo (n=246). Acute response endpoints assessed: 1) Time to first significant response during days 1-7 as measured by Patient Global Assessment Likert scale, 2) Western Ontario and McMaster Osteoarthritis Index (WOMAC) pain subscale using a visual analog scale (VAS) during days 1-7, and 3) American Pain Society Patient Outcome Questionnaire (APS-POQ) scores over the first 7 days. Endpoints to assess sustainability of naproxen/esomeprazole included: 1) Routine Assessment of Patient Data (RAPID)-3 and 2) WOMAC Stiffness, Total and Pain VAS at 6/12 weeks. Time to first significant response was analyzed using the log-rank test and estimated using the Kaplan-Meier analysis method. Other endpoints were analyzed using ANCOVA models with baseline values as covariates. Least square means, confidence intervals, and p-values were generated on change from baseline differences compared to placebo. Effect sizes (Cohen’s D) and correlation coefficients (Spearman, Pearson) were estimated descriptively.
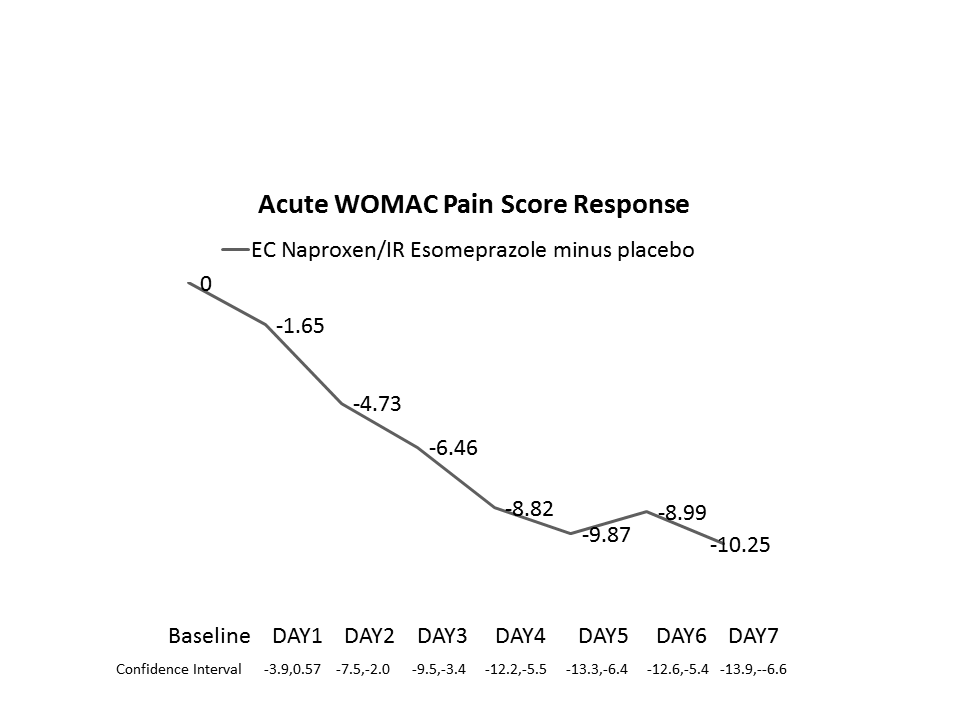
Results: EC Naproxen/IR esomeprazole produced statistically significant decreases in WOMAC Pain on Days 2-7 (Figure) and at Weeks 6 and 12; APS-POQ pain assessments were significantly improved on Days 2-7. RAPID and WOMAC Total/Pain/Stiffness scores decreased significantly at Weeks 6 and 12. Responses were comparable to celecoxib. Pain relief effect sizes were moderate and median days to good-excellent response was 6 days. Total RAPID-3 to WOMAC and WOMAC to RAPID Pain were highly correlated with each other (correlation > 0.80) at 6 and 12 weeks.
Conclusion: EC naproxen/IR esomeprazole produces a moderate-large early pain response which is maintained for 12 weeks. RAPID-3 was found to be highly correlated with the typical OA measure (WOMAC) and might be a useful clinical tool for measuring NSAID response.
(1) Jordan, et. al. J Rheumatol 2007;34(1):172-180.
(2) Hochberg, et. al. Curr Med Res Opin 2011; 27:1243–53.
(3) Cryer, et. a. Ann Med 2011;43:594-605.
Disclosure:
J. Fort,
Pozen, Inc,
3;
R. Holt,
Horizon Pharma, Inc,
5,
Pozen, Inc.,
5;
A. Y. Grahn,
Horizon Pharma, Inc,
3;
J. Steinmetz,
Horizon Pharma, Inc,
5;
Y. Zhang,
Pozen, Inc,
5;
J. Kent,
Horizon Pharma, Inc,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/onset-magnitude-and-durability-of-pain-relief-in-patients-with-knee-oa-receiving-a-fixed-dose-combination-tablet-of-enteric-coated-ec-naproxen-plus-immediate-release-ir-esomeprazole-magnesi/