Session Information
Title: Systemic Lupus Erythematosus - Clinical Aspects and Treatment: Treatment and Management Studies
Session Type: Abstract Submissions (ACR)
Background/Purpose: Monthly (q4w) intravenously (IV) administered belimumab 10 mg/kg is approved for the treatment of adults with active, autoantibody-positive SLE receiving standard therapy. The present analysis was conducted to predict a safe and efficacious weekly (qw) belimumab subcutaneous (SC) dose to be evaluated in a SC Phase 3 trial in adult SLE patients.
Methods: The belimumab IV Phase 3 clinical trials BLISS-52 and BLISS-76 indicated better efficacy with comparable safety for the 10 mg/kg versus the 1 mg/kg dose. A Phase 1 study (BEL114448: NCT#01583530) evaluated the pharmacokinetics (PK) of SC belimumab in healthy subjects as single or multiple doses up to 240 mg. IV and SC PK parameters were estimated using linear, 2-compartment PK models with NONMEM and Pharsight Phoenix modeling platforms, respectively, and chronic exposure profiles were simulated. The target steady-state SC exposure was set to an exposure level approximating the steady-state average belimumab serum concentration (Cavg) for 10 mg/kg IV q4w. Body-size dependent individual clearance values from the population PK analysis of the IV Phase 3 trials were used to predict the range of Cavg for the SC regimen.
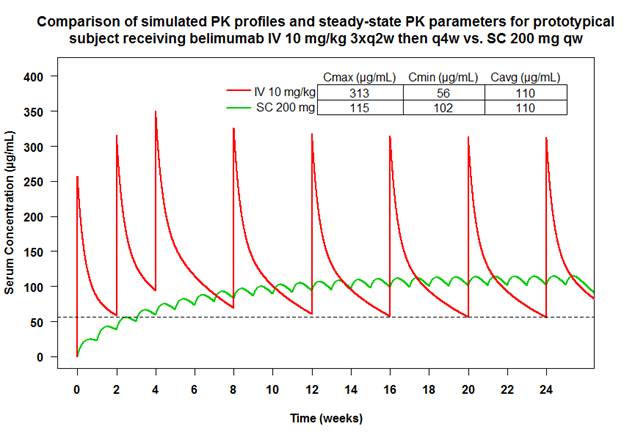
Results: Simulation predicted that belimumab Cavg for 200 mg SC qw closely matched the corresponding Cavg for 10 mg/kg IV q4w. The simulated SC profile showed smaller fluctuations compared to the IV regimen, due to the slow absorption and more frequent dosing. At the end of the first month the trough concentration (Cmin) for 200 mg SC dosing is expected to exceed the steady-state Cmin for the 10 mg/kg IV regimen and therefore a loading dose was not deemed necessary.
The predicted body-size dependent steady-state Cavg values for the SC regimen, demonstrated that the Cavg ranges are similar between 10 m/kg IV q4w and 200 mg SC qw dosing. While for the IV regimen patients with large BMI experienced higher exposure, for the SC regimen higher weight patients are predicted to experience lower exposures.
Conclusion: 200 mg SC qw belimumab is predicted to result in a steady-state belimumab Cavg comparable to the Cavg for 10 mg/kg IV q4w dosing. The fixed SC dose is predicted to result in a similar range of Cavg values as compared to the weight-proportional 10 mg/kg IV dose, albeit with an inverse relationship between body size and exposure. This analysis supported the choice of the belimumab dose tested in the BLISS-SC Phase 3 trial.
Disclosure:
H. Struemper,
GlaxoSmithKline,
1,
GlaxoSmithKline,
3;
D. Roth,
GlaxoSmithKline,
3,
GlaxoSmithKline,
1;
D. Gordon,
GlaxoSmithKline,
1,
GlaxoSmithKline,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predicted-chronic-exposure-and-dose-selection-for-belimumab-administered-subcutaneously-to-sle-patients/