Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The response to treatment of lupus nephritis is unpredictable. There is a need to identify clinical and biochemical characteristics that can predict treatment outcome in lupus nephritis. To do this, we utilized data from the Abatacept and Cyclophosphamide Combination: Efficacy and Safety Study (ACCESS) to identify predictors of renal response at 6 months in patients with lupus nephritis.
Methods: 134 subjects with class III or IV lupus nephritis were randomized to low-dose intravenous cyclophosphamide (IVC) or low-dose IVC with abatacept. Renal response was assessed at 24 weeks. Complete renal response (CR) was defined as: urine protein-to-creatinine ratio (UPCR) <0.5; serum creatinine (Cr) normal, or if abnormal, within 25% of baseline; and adherence to steroid taper regimen. Partial renal response (PR) was defined as: >50% improvement in UPCR; with the same parameters for serum Cr and steroid taper as CR. For the purposes of this analysis, we defined renal response as a composite of CR and PR. We identified possible predictors of renal response, including baseline demographic, clinical, laboratory, and histologic characteristics, as well as clinical and laboratory data obtained within the first 3 months of therapy. We calculated univariate odds ratios (ORs) and 95% confidence intervals (CIs) for renal response for each putative predictor, in the sample as a whole and within each treatment arm. We then conducted a multivariable logistic regression analysis, including all significant predictors (defined as p<0.05) from the univariate regressions.
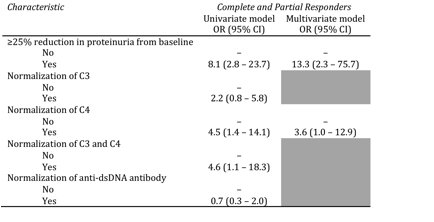
Results: Reduction in proteinuria by at least 25% by week 12 was the strongest predictor of CR or PR at week 24 (OR 8.1; p<0.05). Normalization of C4 and normalization of C3 and C4 by week 12 were also predictive of renal response at week 24 (ORs 4.5 and 4.6 respectively; p<0.05). Reduction in proteinuria by at least 25% and normalization of C4 remained significant independent predictors in the multivariate analysis (ORs 13.3 and 3.6 respectively; p<0.05). This was independent of the treatment arm. None of the baseline characteristics was predictive of renal response.
Conclusion: This study demonstrates that a reduction of at least 25% in proteinuria at 3 months and normalization of C4 levels at 3 months independently predict renal response to therapy with low-dose IVC, with or without abatacept, at 6 months in patients with lupus nephritis. This supports previous findings from the Aspreva Lupus Management Study (ALMS), although reduction in proteinuria was a stronger predictor in this analysis. In contrast to the ALMS analysis, we did not find that time since lupus nephritis diagnosis or baseline eGFR were predictors of renal response. Future studies should address these and other, novel biomarkers so that we can more accurately predict which patients will respond well to treatment.
Disclosure:
S. Goglin,
None;
D. Wofsy,
None;
M. G. Cisternas,
None;
M. Dall’era,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reduction-in-proteinuria-and-normalization-of-c4-complement-levels-predict-response-to-treatment-of-lupus-nephritis-with-low-dose-pulse-cyclophosphamide-and-abatacept/