Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
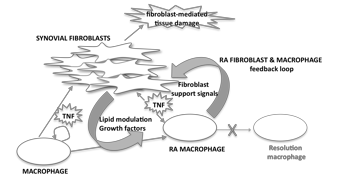
Macrophages and synovial fibroblasts represent key cellular drivers of RA. The goal of this study was to define how the complex cellular programs of macrophages and fibroblasts crossregulate within an inflammatory setting and potentially perpetuate pathologic responses. Previously, we have shown that soluble synovial fibroblast products suppress the macrophage TNF-induced Type I Interferon response. Here through a next-gen sequencing transcriptome analysis, we describe on a global scale how the macrophage TNF-a response is reshaped by synovial fibroblast factors, ultimately converting classic anti-inflammatory/resolution pathways into programs that feedback to support fibroblast growth and function. Furthermore, macrophages isolated directly from RA synovium indeed activate a subset of these gene expression networks, suggesting these macrophage-fibroblast crosstalk pathways represent novel therapeutic targets.
Methods
Human blood derived macrophages and human RA synovial fibroblasts were co-cultured in transwell plates and treated with TNF for 2 days. The transcriptomes of both cell types were analyzed by RNA sequencing (RNAseq). Pathway analysis programs identified macrophage responses specifically controlled by synovial fibroblasts, as well as identified candidate soluble crosstalk mediators. Cellular STAT3 activity was monitored by Western blot detection of phospho-STAT3 levels and by qPCR analysis of STAT3-dependent genes, while the soluble mediators responsible for inducing differential STAT3 responses were confirmed with neutralizing antibodies in the culture media.
Results
Synovial fibroblasts modified specific programs within the macrophage inflammatory response, impacting 30% of TNF-regulated genes. Pathway analysis demonstrated fibroblast factors largely transformed macrophage lipid programs and induced growth factor responses, including genes known to promote wound healing activities and growth of fibroblasts. Interestingly, while TNF-a alone induced an anti-inflammatory STAT3 response, TNF-a combined with fibroblast factors blocked this resolution response and instead diverted STAT3 activity towards mitogenic and metabolic pathways. Furthermore, initial analysis of synovial macrophages isolated directly from RA synovium revealed a subset of the crosstalk pathways identified in the in co-culture system including specific growth factor responses.
Conclusion
We propose the combination of inflammatory and fibroblast-derived factors found in the RA synovium drive macrophages into an unresolving novel macrophage phenotype that in part functions to feedback and support synovial fibroblast pathologic activity.
Disclosure:
L. T. Donlin,
None;
J. Ding,
None;
L. B. Ivashkiv,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/macrophage-fibroblast-crosstalk-pathways-amplify-ra-joint-pathology/