Session Information
Title: Epidemiology and Public Health (ACR): Rheumatoid Arthritis and Systemic Lupus Erythematosus Outcomes
Session Type: Abstract Submissions (ACR)
Background/Purpose: The treatment of juvenile systemic lupus erythematosus (jSLE) often requires complex medication regimens in order to address the different disease manifestations. Despite the limited number of medications approved for the treatment of jSLE, standardized treatment protocols have been slow to emerge. Identification of regional and temporal variations in medication usage in jSLE can help to establish more unified treatment practices.
Methods: Demographic and clinical data were collected for jSLE patients (diagnosis <18 years of age) enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry between 2009 and 2012. Individuals identified as being diagnosed and starting treatment prior to 2007 (Cohort 1; C1; n = 285) or as being diagnosed and treated between 2007 and 2012 (Cohort 2; C2; n = 284) were used to evaluate temporal changes in treatment practices by region. Regions were identified based on U.S. Census definitions and inclusion in a region was determined by the U.S. Census 3-digit zip code tabulation area (ZCTA) prefix of the area in which the patient resided at the time of symptom onset.
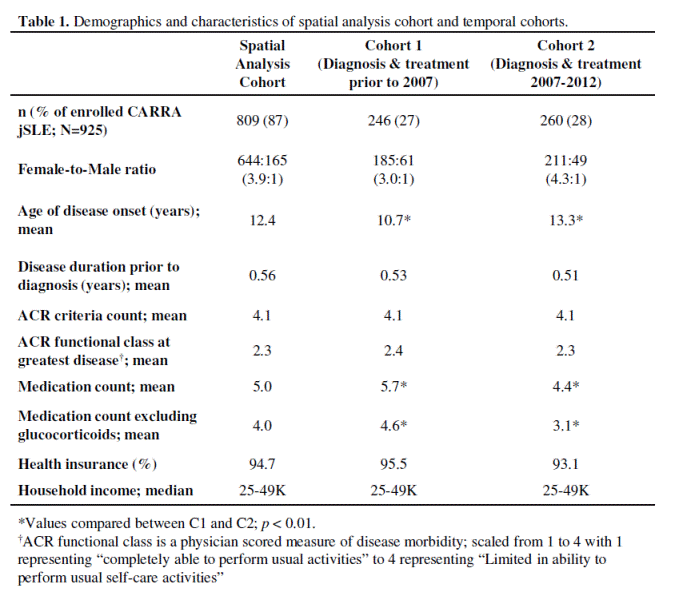
Results: Of the 925 jSLE enrolled in the CARRA Registry by July 2012, 809 (87%) had geographic data available and were included in the spatial analysis. This cohort was further separated based on year of diagnosis and duration of jSLE treatment, resulting in groups C1 and C2. Although the population distribution of jSLE varied among U.S. Census regions, C1 and C2 population sizes within a region were similar. Both demonstrated comparable female-to-male ratios (~3-4:1), ACR criteria counts, and ACR functional scores at worst disease (Table 1).
At symptom onset, approximately 90% of CARRA Registry patients lived in ZCTAs within 50 miles of a CARRA Registry pediatric rheumatology center. Glucocorticoid, non-biologic, biologic, and NSAID use in jSLE varied by region, however, comparison of C1 and C2 suggests an overall decrease in steroid and NSAID use and an increase in use of biologic and non-biologic medications (Fig 1). Furthermore, C2 jSLE required significantly fewer medications compared to C1 (4.4 vs 5.7, respectively; p<0.01).
Conclusion: The heterogeneous nature of jSLE and regional variation in medication usage may have impacted the development of standardized treatment practices. Importantly, however, the overall number of required medications, glucocorticoids and NSAIDs in particular, has decreased across the U.S. Subsequently, regional and temporal variations may reflect the recent trend towards the standardization of medication practices in jSLE.
Disclosure:
J. M. P. Woo,
None;
O. J. Rullo,
None;
D. K. McCurdy,
None;
T. CARRA Registry Investigators,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/u-s-geographic-trends-in-the-distribution-and-treatment-practices-of-juvenile-systemic-lupus-erythematosus-an-analysis-of-the-childhood-arthritis-and-rheumatology-research-alliance-registry/