Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
The optimal strategy to use biologics after rituximab (RTX) in RA is unknown. We therefore aimed to evaluate the effectiveness of different biologics after RTX treatment.
Methods
The CERRERA registry, a prospective, longitudinal, multinational database of RA patients (Pts) treated with RTX was analyzed with respect to the effectiveness of tumor necrosis factor alpha inhibitor (TNFi), abatacept (ABA) or tocilizumab (TCZ) as a new biologic after RTX. Pts were included, if they had stopped RTX no longer than 6 months prior to the new biologic, had a baseline (BL) visit within 21 days of commencement of the new biologic, and at least 1 follow up visit. BL characteristics were compared across biological classes and between TCZ mono and TCZ combination therapy (methotrexate (MTX) and/or leflunomide (LEF)).
Results
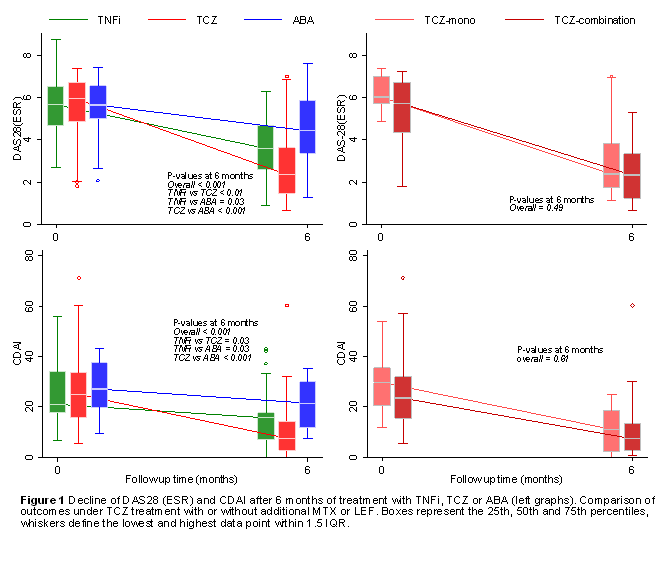
The inclusion criteria were met by 265 Pts. Demographic and disease characteristics did not differ across treatment groups (Table). Pts on TCZ had a significantly greater decline of DAS28(ESR) and CDAI, than Pts on TNFi or ABA after 6 months of treatment (Figure 1). This effect was also seen after adjusting for prednisone use and the number of previous biologics. DAS28 scores in Pts on TCZ were 1.0 (95% CI 0.2-1.7) and 1.8 (95% CI 1.1-2.6) lower than in Pts on TNFi or ABA, respectively. Similarly, the CDAI was 7.2 (95% CI 0-15) and 7.5 (95% CI 0-15) points lower in Pts on TCZ than on TNFi or ABA. Pts with TCZ more frequently had a good EULAR response than Pts with TNFi or ABA (66% vs 31% & 14%, p<0.001). Overall, Pts reported a lower HAQ after 6 months of treatment (p<0.001), though the decline did not differ between treatment groups.
When comparing TCZ mono with TCZ combination therapy, ΔDAS28 and ΔCDAI and EULAR response did not differ. The drug retention rates did not differ between all treatments (Figure 2).
Conclusion
In this observational cohort study, TCZ provided a better control of RA activity than ABA or TNFi in Pts who discontinued RTX. There was no difference in effectiveness between TCZ given as mono therapy and TCZ given in combination with non-biologic DMARDs.
Disclosure:
U. A. Walker,
Roche, UCB, MSD, Pfizer,
8;
V. K. Jaeger,
None;
K. Chatzidionysiou,
None;
M. L. Hetland,
None;
E. M. Hauge,
None;
K. Pavelka,
MSD, AbbVie, Pfizer, UCB, Roche, Amgen, Menarini, BMS,
5;
D. C. Nordström,
Roche Pharmaceuticals,
9;
H. Canhao,
Abbvie, MSD, Pfizer, Roche and UCB,
9;
M. Tomsic,
Roche Pharmaceuticals,
9;
R. van Vollenhoven,
AbbVie, BMS, GSK, Pfizer, Roche, UCB,
2,
AbbVie, Biotest, BMS, GSK, Janssen, Lilly, Merck, Pfizer, Roche, UCB, Vertex,
5;
C. Gabay,
Roche, Merck, and Abbvie,
2,
Roche, Abbvie, Pfizer, BMS, Sanofi-Aventis, Merck, AB2 Bio,
8,
Roche, Abbvie, Pfizer, BMS, Sanofi-Aventis, Merck, AB2 Bio,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rituximab-done-whats-next-in-ra/