Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
Topically applied non-steroidal anti-inflammatory drugs (NSAIDs) can produce clinically effective drug concentrations at a peripheral site, but with low systemic concentrations and thus a lower risk of AEs. Various factors influence penetration and absorption of topical NSAIDs such as chemical properties of adjuvants included in the formulation and inter-individual variability in skin absorption. Modulating skin permeation is a pivotal factor in the development of new formulations as well as their posology and duration of therapeutic effect. Diclofenac diethylamine (DDEA) 1.16% gel (Voltaren® EmulgelTM [VEG 1%], Novartis Consumer Health, Nyon Switzerland) is used topically to relief pain and inflammation; it is applied to the affected site 3 or 4 times daily. We compared the in vitro skin permeation of VEG 1% with a new double-strength formulation of DDEA 2.32% [VEG 2%] including different doses of a permeation enhancer (PE) to assess the hypothesis of increased skin permeation that may result in delivering higher drug amounts to target tissues when applied every 12h.
Methods
In vitro skin penetration studies were performed at 35°C in glass Franz static diffusion cells (Bernard Gallas, Antibes, F; 1.54 cm2) using human skin. Samples of donor abdomens were obtained from the NDRI Institute (USA) and the WHRTB Institute (HU) and kept frozen at –80°C until use. The comparison of in vitro skin permeation of VEG 1% vs VEG 2% was performed. Then VEG 1% was tested against VEG 2% containing low (0.5%), medium (0.75%) and high (1.0%) amounts of a permeation enhancer. All samples were applied at 20 mg/cm2 in a single dose, equivalent to the maximum daily dose of 4 applications of 5 mg/cm2 under in use conditions.
Results
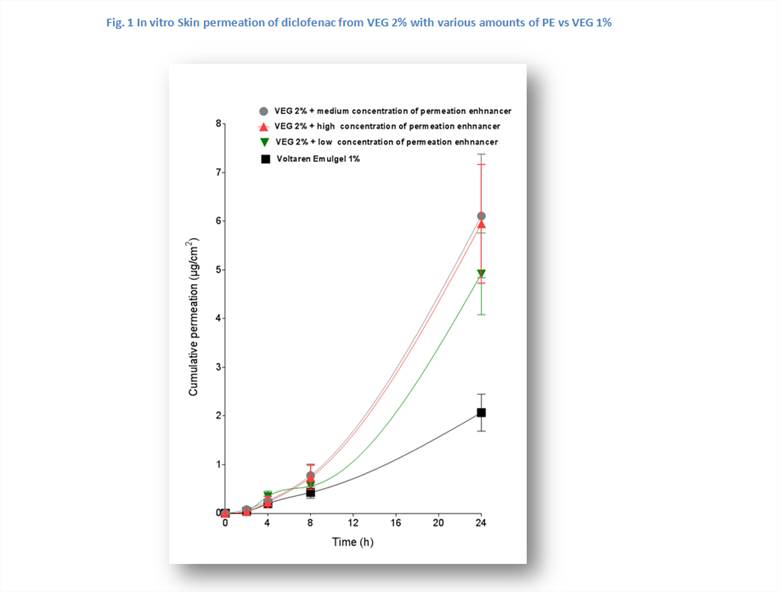
Addition of the PE at the VEG 2% gel resulted at a dose-dependent (up to 3-fold) increase in diclofenac’s skin permeation (Fig. 1). Medium and high PE concentration resulted in similar cumulative permeation.
Conclusion
Addition of PE to VEG 2% resulted in an up to 3-fold increased diclofenac skin permeation which correlated with the PE dose concentrations. The new gel is expected to have a lasting effect, e.g. up to 12h, potentially attributed to the release of a higher amount of drug at the application site as confirmed in a RCT (Predel 2012), where 2 daily doses significantly reduced pain and improved joint function vs placebo. This could be useful when treating flares of peripheral joint conditions such as OA.
Disclosure:
G. Quartarone,
None;
N. Hasler-Nguyen,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-between-two-diclofenac-diethylamine-gel-formulations-1-16-vs-2-32-is-it-only-increasing-the-strength-of-the-active-ingredient-enough/