Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Bio-lubrication is a prerequisite for proper joint mobility and is crucial for prevention of degradative changes of the joint. Phospholipids are components of the synovial fluid and are known to serve as natural lubricants of cartilage surfaces. MM-II is a novel intra-articular bio-lubricant made of liposomes suspended in aqueous solution.
Purpose: to test the safety and effectiveness of intra-articular injection of MM-II in osteoarthritic patients compared with intra-articular hyaluronic acid (HA) up to 3 months of follow-up in a double-blind, randomized clinical study.
Methods: Patients with symptomatic unilateral knee OA with baseline pain on VAS of more than 40 mm and a stage 2-3 Kellgren Lawrence score on X-ray, were recruited. 40 patients were randomized into two groups of 20, to receive a single intra-articular injection of either MM-II or HA (Durolane®). Effectiveness measures included maximal global pain in the target knee, recorded by a 100mm VAS; WOMAC subscales; OMERACT OARSI responder criteria; PGA, PASS, PAE questions and consumption of paracetamol/acetaminophen, which was the only authorized rescue medication. Tolerability was assessed by local manifestation defined by an increase of at least 3 cm in knee circumference, measured at 2 cm above the upper border of the patella or local pain increase of more than 30 mm on a 100 mm VAS. Adverse events were recorded through 90-days of follow-up.
The study was FIM Exploratory non-powered, with descriptive statistics.
Results: All patients completed the study.
Results relating to WOMAC A pain, summarized in Figure 1, show a faster response with MM-II, with maximal effect observed on day 14, which was maintained over time and was associated with a statistically significant difference from baseline pain from day 7. In the HA group, the onset of pain relief was slower, with an improvement statistically significant change from baseline observed only on day 90.
Daily acetaminophen intake was lower in the MM-II group, with a reduction of more than 50% in the number of days and total dose of rescue medication consumption seen following MM-II administration, compared with HA injection.
The percent of responders to treatment according to the OMERAC-OARSI response criteria was 52.6, 66.7, 70 & 60 at the day 7, 14, 30 & 90 respectively compared to 30, 36.8, 25, 45 at the HA group (data not shown).
Local adverse events (inflammatory flare) were observed in one patient at day 3 in the MM-II group and in 4 pts at day 1, 1 pt at day 3, and 1 pt at day 7 in the HA group.
Patient’s Relative Change in WOMAC A in Target Knee over Time
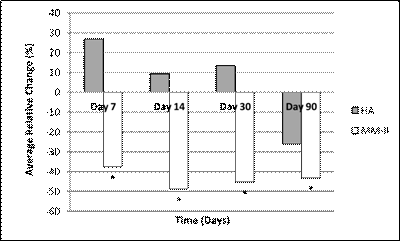
*statistically significant difference from baseline
Conclusion: Intra-articular injections of MM-II were found to be safe and effective. The pain-reduction action was more rapid and sustained up to 3 months compared with HA. Larger randomized controlled trials are needed to confirm these encouraging results.
Disclosure:
L. Kandel,
MM-II ,
2;
Y. Dolev,
Stock options,
1;
R. Shimonov,
None;
G. Rivkin,
None;
M. Liebergall,
None;
Y. Mattan,
None;
X. Chevalier,
MM-II,
6.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-liposome-intra-articular-injection-in-moderate-knee-osteoarthritis-a-prospective-randomized-double-blinded-study/
