Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Fatty lesions of the bone marrow in the axial skeleton (sacroiliac joints – SIJ, and spine) on magnetic resonance imaging (MRI) are considered nowadays as earliest post-inflammatory changes preceding new bone formation in axial spondyloarthritis (axSpA). It has been shown in several trials with tumour necrosis factor (TNF) α inhibitors that resolution of inflammation under anti-TNF therapy is associated with an increase of a fatty lesion score. This raised concerns that TNF blockers might therefore promote the process of new bone formation in axSpA. The aim of the current analysis was to investigate the difference in fatty lesions formation rates in patients treated with the TNF inhibitor infliximab (IFX) added to naproxen (NPX) as compared to NPX alone given over 28 weeks in patients with early axSpA.
Methods: Part I of the INFAST study was a double-blind, randomized controlled trial of IFX in biologic-naïve patients 18–48 years of age with early (<3 years symptom duration), active axSpA with signs of active sacroiliitis on MRI. A total of 158 patients were randomized (2:1) to receive 28 weeks of treatment with either intravenous IFX 5 mg/kg (weeks 0, 2, 6, 12, 18, and 24) + NPX 1000 mg/d (n=106) or intravenous PBO+NPX 1000 mg/d (n=52). MRIs of the SIJ and of the spine were performed at baseline and week 28 and were scored according to the Berlin scoring system for active inflammation and for fatty lesions, including a detailed fatty degeneration score for the SIJ by a reader who was blinded for clinical data including treatment allocation.
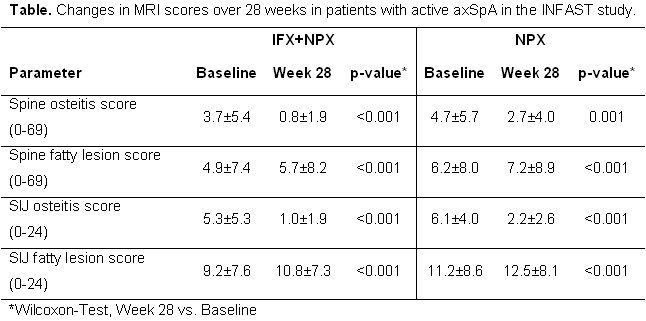
Results: Complete MRI sets (baseline and week 28, both STIR and T1-weighted sequences) were available in 147 patients for the spine (n=99 IFX+NPX, n=48 NPX alone) and in 143 patients for the SIJ (n=97 IFX+NPX, n=46 NPX alone). At baseline there were no meaningful differences between treatment group neither in osteitis nor in fatty lesion scores. In both treatment groups there was a significant reduction of inflammation in the spine and in the SIJ at week 28 as compared to baseline – table, which was however more prominent in the combined treatment group since after 28 weeks patients in the IFX+NPX group had significantly lower osteitis scores in the SIJ (p=0.001) and in the spine (p<0.001). Similarly, in both groups there was a significant and comparable increase in the fatty lesion score (table) in the spine and in the SIJ at week 28 as compared to baseline, but no statistically significant difference between treatment groups was observed in the fatty lesion status score at week 28.
Conclusion: Effective anti-inflammatory treatment of axSpA in this study was associated with an increase in the fatty lesion score in the SIJ and in the spine that was independent of the treatment arm. The results suggest that fatty lesion formation after resolution of inflammation is possibly a universal pathogenetic mechanism in axSpA and not a direct effect of anti-TNF therapy.
Disclosure:
D. Poddubnyy,
Abbvie ,
5,
MSD,
5,
Pfizer Inc,
5,
UCB,
5,
Novartis Pharmaceutical Corporation,
5,
Janssen Pharmaceutica Product, L.P.,
5;
J. Sieper,
Abbott, Merck, Pfizer, UCB Pharma, Novartis, Lilly, Janssen,
5,
Abbott, Merck, Pfizer, UCB Pharma, Novartis, Lilly, Janssen,
8.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/infliximab-added-to-naproxen-does-not-increase-frequency-of-new-fatty-lesions-on-mri-of-the-sacroiliac-joints-and-of-the-spine-as-compared-to-naproxen-alone-in-early-axial-spondyloarthritis/