Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Fostamatinib (Fosta) is a novel spleen tyrosine kinase (SYK) inhibitor. The Phase II TASKi studies showed benefit in patients (pts) with active rheumatoid arthritis (RA). A formal, prospective, international, phase III research program (OSKIRA) was initiated in 2010. This 52-wk study, (OSKIRA-2) evaluated Fosta in pts with active RA who had an inadequate response to DMARDs.
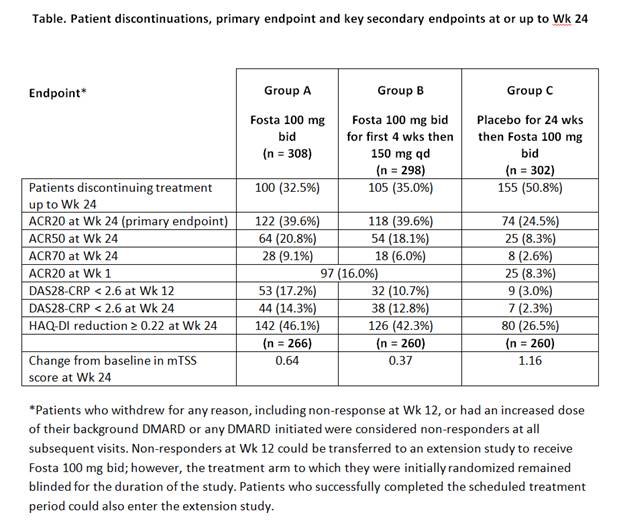
Methods: 908 pts who fulfilled 1987 ACR criteria were randomly allocated to 1 of 3 groups: Fosta 100 mg bid (Group A, n = 308); Fosta 100 mg bid for 4 wks then Fosta 150 mg qd (Group B, n = 298); or placebo (PBO) for 24 wks then Fosta 100 mg bid (Group C, n = 302). All treatment regimens were taken in combination with DMARDs. Non-responders at Wk 12 could leave the study and enter an active extension. The primary endpoint was the proportion of pts achieving ACR20 at Wk 24. Secondary endpoints evaluated efficacy, safety and tolerability up to 52 wks.
Results : There were no notable imbalances in demographics, disease duration and activity/disability/erosions or concomitant medications at baseline between the 3 groups (mean age: 53 yrs; 82% female; mean disease duration: 7.5 yrs; RF seropositive: 78%; erosive score ≥ 3: 80%; mean DAS28-CRP score: 5.6). Background medications included methotrexate (83% of pts) and sulfasalazine, hydroxychloroquine or chloroquine (17% of pts). There were statistically significant improvements in ACR20 at Wk 24 for both Fosta groups vs PBO (p < 0.001 for both groups; Table). For all the key secondary endpoints evaluating RA signs and symptoms up to Wk 24, Fosta Group A achieved statistically significant differences vs PBO (p < 0.001 for all; Table) and there were statistical differences from PBO for these endpoints for Group B (p < 0.001), except ACR70 (Table). Changes in ACR20 were seen as early as Wk 1. Change in mTSS at Wk 24 did not show a significant difference compared with PBO for either Fosta group (p = 0.904; p = 0.342, respectively).
The most frequently reported AEs in Fosta-treated pts included hypertension, nasopharyngitis, diarrhea and hepatic enzyme increases. No major differences in serious AEs were encountered between the 3 groups (6.8%, 4.7% and 3.3% of pts, respectively). As expected, patients developed elevated blood pressure with Fosta, with elevated BP (≥ 140/90 mmHg) observed in 49.8% and 48.1% (Groups A and B), vs. 29.5% of pts (PBO) at ≥ 1 visit during the first 24 wks.
Conclusion : Fosta had an early beneficial effect on signs and symptoms, disease activity and physical function in RA vs. PBO. The overall level of response with Fosta was not as large as observed in the phase ll (TASKi) program. Treatment at the higher dose (100 mg bid) generally showed a more consistent improvement in signs and symptoms. However, Fosta failed to show benefit in mTSS. Safety and tolerability findings were consistent with the profile observed in earlier Fosta studies.
Disclosure:
P. Dawes,
Haywood Foundation,
2,
AstraZeneca,
5;
A. Dimic,
AstraZeneca,
9;
M. C. Genovese,
Rigel Pharma,
2,
Rigel Pharma,
5,
AstraZeneca,
2,
AstraZeneca,
5;
D. van der Heijde,
AstraZeneca,
5,
AbbVie,
5,
Amgen,
5,
Augurex,
5,
Bristol-Myers Squibb,
5,
Celgene,
5,
Centocor, Inc.,
5,
Chugai,
5,
Covagen,
5,
Daiichi Pharmaceutical Corporation,
5,
Eli Lilly and Company,
5,
GlaxoSmithKline,
5,
Janssen Biologics,
5,
Merck Pharmaceuticals,
5,
Novartis Pharmaceutical Corporation,
5,
Novo-Nordisk,
5,
Otsuka,
5,
Pfizer Inc,
5,
Roche Pharmaceuticals,
5,
Sanofi-Aventis Pharmaceutical,
5,
Schering-Plough,
5,
UCB,
5,
Vertex,
5,
Imaging Rheumatology BV,
9;
M. Jenkins,
AstraZeneca,
1,
AstraZeneca,
3;
C. O’Brien,
AstraZeneca,
1,
AstraZeneca,
3;
B. Oemar,
AstraZeneca,
3;
J. Vencovsky,
None;
M. Weinblatt,
Rigel Pharma,
5,
AstraZeneca,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/oskira-2-a-phase-iii-multicenter-randomized-double-blind-placebo-controlled-parallel-group-study-of-2-dosing-regimens-of-fostamatinib-in-rheumatoid-arthritis-patients-with-an-inadequate-response/