Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Frequently DAS28-CRP is utilized instead of DAS28-ESR to assess rheumatoid arthritis (RA) disease activity; however, values for remission and low disease activity (LDA) for DAS28-CRP have not been validated.1,2 Differences between remission rates could vary between 8–24% and therefore, the cut-off points should not be identical for the two measures.3–5 The aim of this analysis is to examine the value of DAS28-CRP, which corresponds to the value of DAS28-ESR for cut-off points from 3 different patient populations.
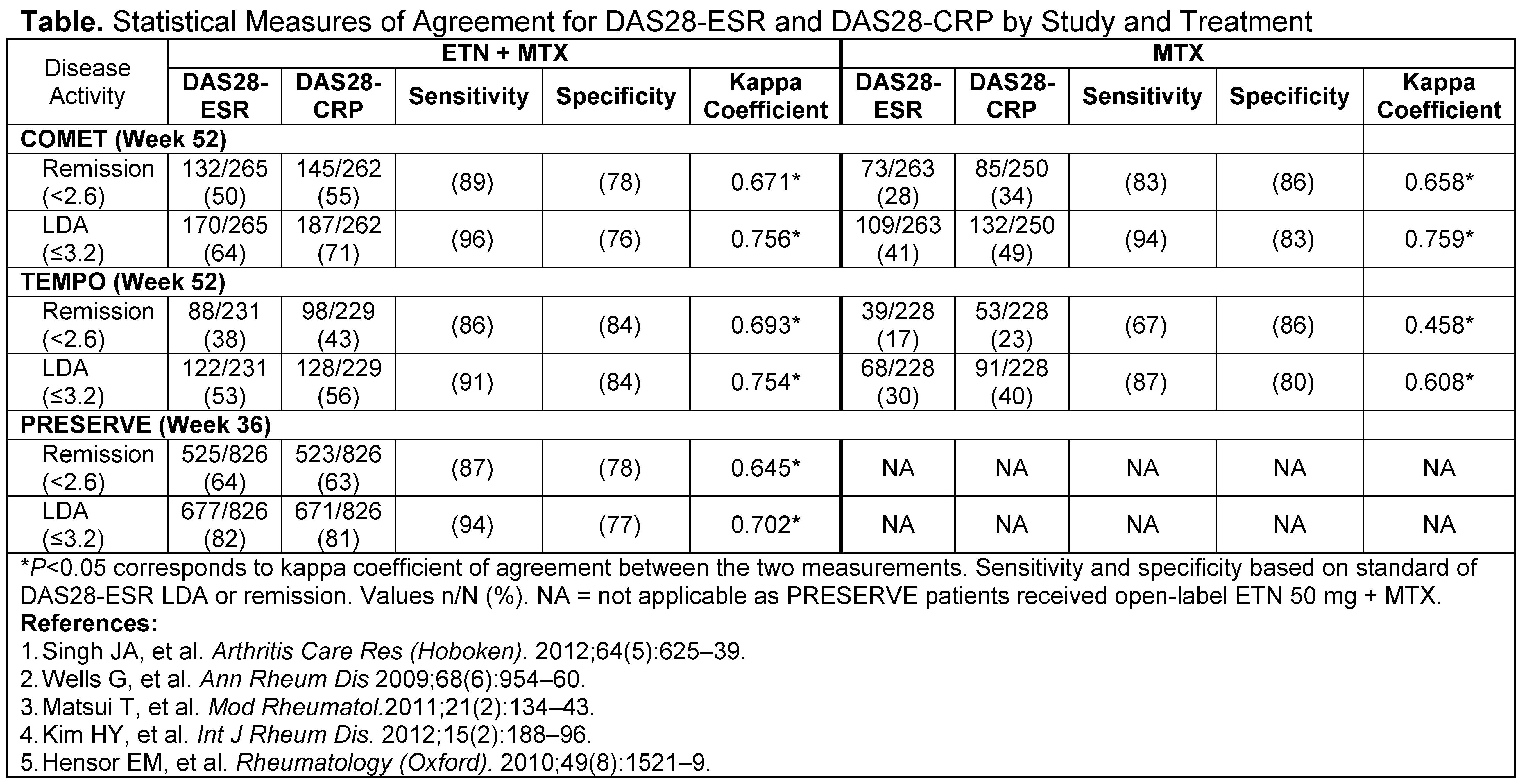
Methods: Randomized patients from COMET (early, moderate-to-severe RA), TEMPO (established moderate-to-severe RA), and PRESERVE (moderate RA) who received etanercept (ETN) 50 mg weekly + MTX or MTX alone were included. DAS28-ESR and DAS28-CRP response rates were compared (COMET, TEMPO week 52; PRESERVE week 36) for each study and by treatment group utilizing the traditional ESR cut-off points for remission <2.6 and LDA ≤3.2; level of agreement was determined by kappa coefficient, sensitivity, and specificity. Remission and LDA cut-offs for DAS28-CRP were based on patients' final time point, with treatment groups and studies being pooled together before utilizing ROC analysis to obtain the new cut-offs that best correspond to ESR established cut-offs. DAS28-ESR and CRP, along with their unique components (0.70*ln[ESR] and 0.36*ln[CRP + 1] + 0.96), were analyzed as continuous parameters using Spearman's correlation. DAS28-CRP cut-offs for the Asian population were examined and compared with the overall population.
Results: The percentage of patients meeting the definition of remission (<2.6) or LDA (≤3.2) was slightly higher for DAS28-CRP than DAS28-ESR for both treatment groups across all 3 trials (Table). The concordance in which patients achieved the same outcome for both DAS28-ESR and CRP LDA or remission ranged from 82–91%. Although, DAS28-ESR and CRP measures were highly correlated (range 0.80–0.90; P<0.001) for each study's final time point, their unique components were not highly correlated (range 0.35–0.50; P<0.001). New DAS28-CRP cut-offs were found to range from 2.4–2.5 for remission and 3.0–3.1 for LDA, depending on study and treatment with 2.52–3.05 providing the best overall results. Cut-offs determined from the Asian population showed some differences, especially LDA, compared with the overall population suggesting regional differences exist.
Conclusion: The percentage of patients achieving remission or LDA was lower for DAS28-ESR than DAS28-CRP when utilizing the same cut-off points for both measures. DAS28-CRP underestimates disease activity when utilizing cut-off points validated for DAS28-ESR and therefore, should be evaluated using different remission and LDA values. Studies are needed to validate proposed DAS28-CRP disease activity cut-offs.
Disclosure:
R. Fleischmann,
Pfizer Inc,
2,
Pfizer Inc,
8;
D. van der Heijde,
Abbott Laboratories, Amgen, Aventis, Bristol Myers Squibb, Centocor, Pfizer, Roche, Schering Plough, UCB, Wyeth ,
5;
A. S. Koenig,
Pfizer Inc,
3,
Pfizer Inc,
1;
R. Pedersen,
Pfizer Inc,
3,
Pfizer Inc,
1;
A. Szumski,
None;
L. Marshall,
Pfizer Inc,
3,
Pfizer Inc,
1;
E. Bananis,
Pfizer Inc,
1,
Pfizer Inc,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-activity-score-28-joint-count-are-erythrocyte-sedimentation-rate-and-c-reactive-protein-versions-comparable/