Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
IgG4-related disease (IgG4-RD) is an immune-mediated disorder that responds to B cell depletion with rituximab (RTX). We detected IgG4+ plasmablasts in the sera of patients with IgG4-RD and observed a general correlation with disease activity. We examined the relationship between disease activity and IgG4+ plasmablast concentration more closely in eight patients treated with RTX.
Methods:
This study was approved by the institutional review board and all subjects provided informed, written consent. All patients had histopathologic proof of their IgG4-RD diagnoses, and their clinical features were consistent with this diagnosis. Patients were selected for rituximab treatment based on previous refractoriness to glucocorticoids or disease activity at one or more organ sites. Rituximab (1000 mg) was administered in two doses at days 0 and 15. Absolute plasmablasts/ml were measured by flow cytometry, gated on IgG4+, CD38+, CD27+ and CD19 lo. Clinical visits and laboratory evaluations were performed at baseline and approximately 3 months later. One subject had the baseline plasmablast concentration measured 2 weeks after RTX, and another had the measurement at 5 months post-RTX treatment. The IgG4-RD responder index (IgG4-RD RI) was used to measure disease activity. Concentrations of plasmablasts before and after RTX were compared using a paired t-test.
Results:
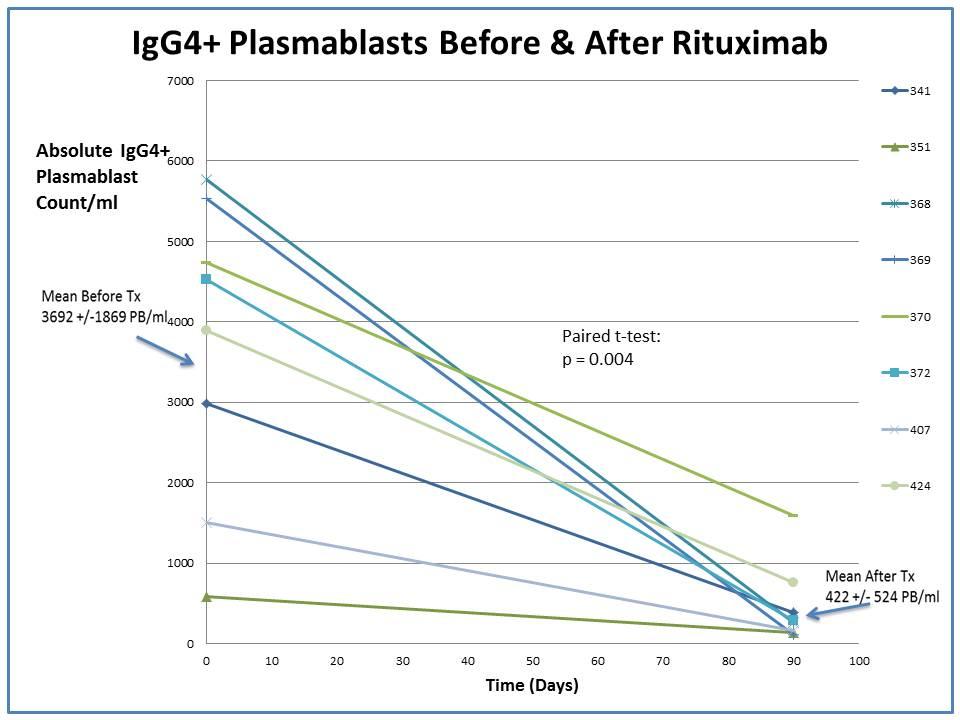
IgG4+ plasmablast concentrations decreased dramatically following RTX (Figure 1). The mean absolute IgG4+ plasmablast counts per ml before and after treatment were 3692 (range: 610 to 5772) and 422 (range: 1 to 156), respectively (p=0.004). The IgG4-RD RI declined in a similar fashion, from a mean of 13±4 to 3±2 (p=0.0001). Three patients had baseline serum IgG4 concentrations <140 mg/dl yet still had substantial blood plasmablast elevations, with a mean of 5279 PB/ml (range: 4530 to 5772). The mean serum IgG4 concentration also decreased significantly following RTX, from 284 mg/dl (range: 58 to 638) to 177 mg/dl (range: 47 to 387 mg/dl)(p=0.025).
Conclusion:
The IgG4+ plasmablast level falls significantly 3 months after treatment with RTX, corresponding to improvements in disease activity in IgG4-RD. IgG4+ plasmablasts may be an important biomarker in IgG4-RD for diagnosis, serial assessment of disease activity, and a gauge for the need for treatment.
Disclosure:
M. Carruthers,
None;
H. Mattoo,
None;
Z. S. Wallace,
None;
V. Mahajan,
None;
S. Pillai,
None;
J. H. Stone,
Genentech and Biogen IDEC Inc.,
2,
Genentech and Biogen IDEC Inc.,
5,
Roche Pharmaceuticals,
2.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/igg4-plasmablasts-are-a-novel-biomarker-in-igg4-related-disease/