Session Information
Session Type: Poster Session
Session Time: 10:30AM-12:30PM
Background/Purpose: In systemic lupus erythematosus (SLE), IgG antibodies that target nuclear antigens (ANA) are pathogenic and part of the diagnostic criteria for SLE. Healthy individuals (HC) possess ANA⁺ mature B cells but these cells do not differentiate into IgG+ plasma cells and do not produce pathogenic antibodies. This study aimed to identify differential activation and regulatory mechanisms of ANA⁺ B cells in patients with SLE and HC. To gain insight into immune homeostasis during remission, we included patients with active disease (SLE-A) and those in remission (SLE-R).
Methods: We profiled the transcriptomes of ANA⁺ and ANA⁻ B cells using single-cell RNA sequencing (scRNA-seq). Samples were obtained from 7 patients with SLE-A, 5 SLE-R, and 7 HC. Three B cell subsets were isolated (ANA⁺ and ANA⁻): (1) CD19⁺ CD27⁻ CD38^int (encompassing naïve B cells and age-associated B cells [ABCs]), (2) CD19⁺ CD27⁺ IgM⁺ (including circulating marginal zone B cells [MZB] and IgM memory B cells), and (3) CD19⁺ CD27⁺ IgG⁺ (IgG memory B cells).
Results: We identified seven transcriptionally distinct B cell clusters defined by canonical lineage and activation markers: naïve, activated naïve, marginal zone B cells (MZB), IgM memory, memory (predominantly IgG⁺), activated memory (also predominantly IgG⁺), and age-associated B cells (ABCs). Minimal overlap in cluster composition was observed between SLE-A and HC, while SLE-R exhibited a unique intermediate profile.
Pathway analysis revealed that activated clusters were enriched for interferon (IFN) and TNF-α and oxidative phosphorylation signatures compared to quiescent clusters. Using a published compendium of scRNA-seq profiles of immune cells in response to cytokines, we confirmed an IFN signature across all SLE patients, with higher expression in SLE-A relative to SLE-R. TNF signaling was consistently present in activated clusters from both SLE-A and SLE-R, with comparable magnitude.
To identify differentially expressed genes in ANA⁺ B cells, we applied a zero-inflated model to pseudobulk data, stratified by cluster and cohort. ANA⁺ B cells showed enrichment of B cell receptor (BCR) signaling pathways, consistent with chronic antigen exposure. Notably, Fc receptor signaling was upregulated in ANA⁺ B cells from HC and SLE-R, but absent in SLE-A.
Conclusion: Our findings highlight the relevance of activated memory B cell subsets and ABCs in SLE pathogenesis, with SLE-R marked by a persistent activated naïve B cell population enriched for TNF signaling. We identified a regulatory checkpoint in ANA⁺ memory B cells that is impaired in SLE-A. Activated B cell clusters in SLE exhibited reduced oxidative phosphorylation, suggesting a metabolic shift toward glycolysis. Differential regulation of Fcgr2b between ANA+ and ANA- in SLE-A suggests that Fcgr2b may modulate ANA⁺ B cell activation in health and remission but is dysregulated during active disease. This further implicates Fc receptor signaling as a potential therapeutic target to promote or sustain remission in SLE.
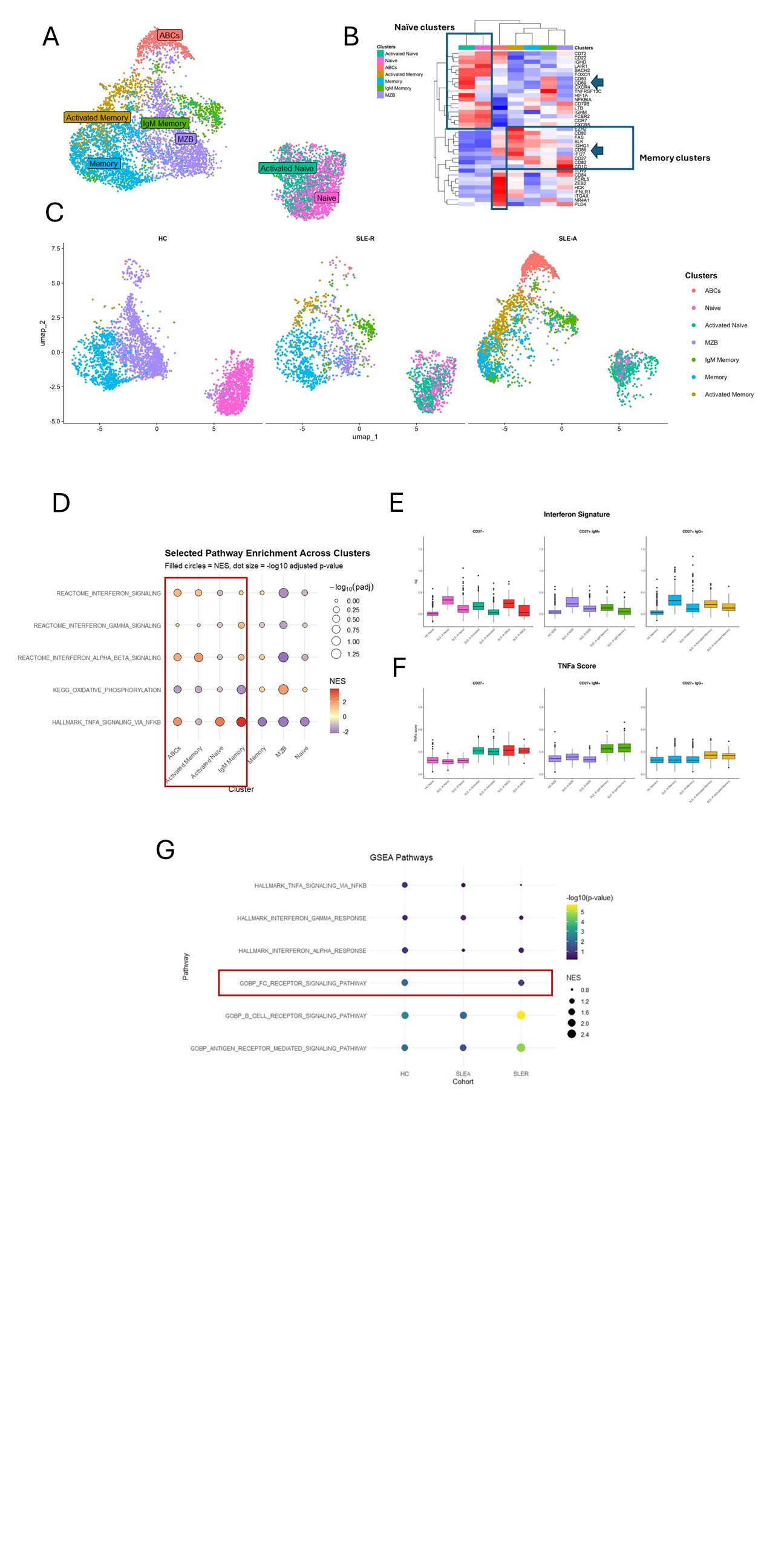 Figure 1. scRNA-seq analysis of ANA+ and ANA- B cells from patients with SLE-A, SLE-R and healthy controls (HC). A. UMAP showing the seven clusters that were identified including B cells from 3 groups. B. Heatmap shows main markers that define each cluster. C. Distribution of cells according to the cohort of origin. D. Selected pathways that are significantly different between clusters. The red square includes the subsets that are enriched in patients with SLE-A. E and F. Interferon and TNFα scores calculated according to the immune dictionary (as described in the main text) for each cluster divided by ANA+ and ANA-. G. Pathway analysis of genes that are significantly different between ANA+ and ANA- B cells for each cohort.
Figure 1. scRNA-seq analysis of ANA+ and ANA- B cells from patients with SLE-A, SLE-R and healthy controls (HC). A. UMAP showing the seven clusters that were identified including B cells from 3 groups. B. Heatmap shows main markers that define each cluster. C. Distribution of cells according to the cohort of origin. D. Selected pathways that are significantly different between clusters. The red square includes the subsets that are enriched in patients with SLE-A. E and F. Interferon and TNFα scores calculated according to the immune dictionary (as described in the main text) for each cluster divided by ANA+ and ANA-. G. Pathway analysis of genes that are significantly different between ANA+ and ANA- B cells for each cohort.
To cite this abstract in AMA style:
Pozovskiy R, Atisha-Fregoso Y, Diamond B. Transcriptomic Signatures of ANA+ and ANA- B Cells Reveal Shifts from Active Disease to Remission in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/transcriptomic-signatures-of-ana-and-ana-b-cells-reveal-shifts-from-active-disease-to-remission-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/transcriptomic-signatures-of-ana-and-ana-b-cells-reveal-shifts-from-active-disease-to-remission-in-systemic-lupus-erythematosus/
