Session Information
Session Type: Poster Session
Session Time: 10:30AM-12:30PM
Background/Purpose: There is a substantial unmet need for effective, well-tolerated therapies for patients with SLE. Deucravacitinib is an oral, selective tyrosine kinase 2 (TYK2) inhibitor approved in moderate to severe plaque psoriasis and under evaluation in phase 3 SLE studies. Deucravacitinib demonstrated superior efficacy vs placebo (PBO) in the phase 2 PAISLEY SLE study. Here, we report integrated analyses from the PAISLEY SLE (parent; NCT03252587) and PAISLEY long-term extension (LTE; NCT03920267) studies.
Methods: Patients completing the 48-week parent study while on study drug (deucravacitinib 3 mg twice daily [BID], 6 mg BID, or 12 mg once daily [QD] or PBO) were enrolled in the 3-year LTE study (Figure 1). Patients on PBO were rerandomized 1:1:1 in the LTE study to 1 of the 3 deucravacitinib doses (blinded), while those already on deucravacitinib continued their regimen for up to 4+ years of treatment (222 weeks). The LTE study allowed greater flexibility in adjustments to background treatment. Databases of the parent and LTE studies were integrated for analyses of safety through W226 and efficacy through W222. Efficacy analyses using composite measures of disease activity were summarized descriptively without formal statistical comparisons; additional efficacy results based on imputation for missing data to minimize survivor bias will be presented. For binary endpoints, 95% CIs were obtained using the Clopper-Pearson method.
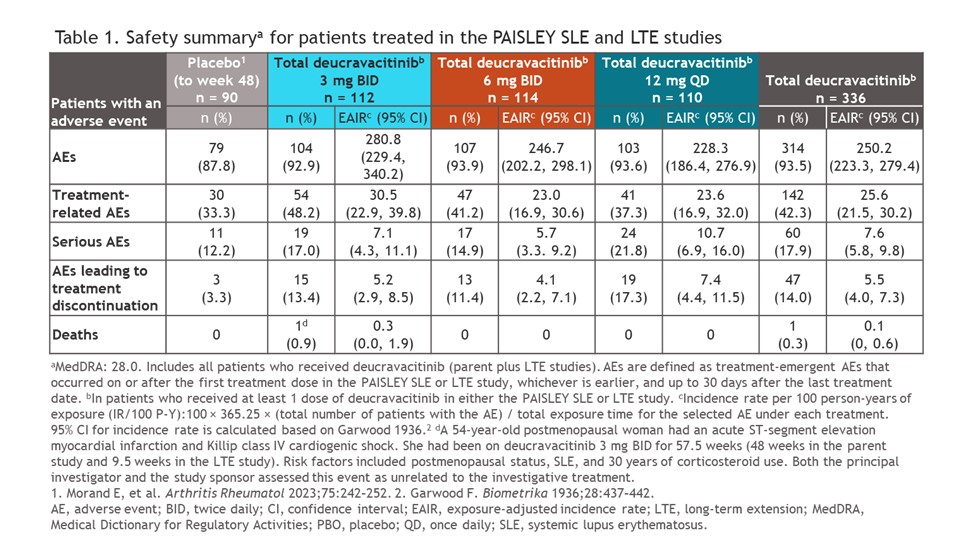
Results: Of 363 patients from the parent study, 261 (72%) were enrolled in the LTE study; 151/261 patients (57.9%) completed the study. Adverse events (AEs) with up to 4+ years of treatment are shown in Table 1; serious AEs occurred in 17%, 14.9%, and 21.8% of patients who received deucravacitinib 3 mg BID, 6 mg BID, and 12 mg QD, respectively, and AEs leading to treatment discontinuation occurred in 13.4%, 11.4%, and 17.3%, respectively. One death deemed unrelated to study treatment occurred in the deucravacitinib 3-mg BID arm. SRI(4), BICLA, LLDAS, and DORIS remission rates were maintained over time (Figure 2); patients in the PBO arm in the parent study who switched to deucravacitinib achieved improvements that were broadly comparable to those of continuously treated patients from ≈W72 (Figure 2). CLASI-50/70 and JC-50/100 responses were maintained consistently throughout the LTE study, with increased responses seen in the PBO-switch arm. The incidence of moderate to severe flares was low across all treatment arms.
Conclusion: Integrated analysis of the PAISLEY SLE and LTE studies confirmed the consistent safety profile and sustained efficacy of deucravacitinib in SLE with up to 4+ years of drug exposure, with no new safety signals despite complex background therapies. Patients who switched from PBO during the LTE study achieved outcomes comparable to those treated from baseline. These data represent the longest follow-up of a TYK2 inhibitor in SLE to date, supporting the potential of deucravacitinib as a durable future treatment option, currently under evaluation in phase 3 POETYK SLE trials (NCT05617677; NCT05620407).
To cite this abstract in AMA style:
Morand E, Arriens C, Pike M, Merrill J, Werth V, Touma Z, Ionitescu R, Okada M, Kouris I, Kolekar Y, Nie J, Renukuntla V, Wegman T, van Vollenhoven R. Four-Year Safety and Efficacy of Deucravacitinib in Systemic Lupus Erythematosus: Results From a Phase 2 Program [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/four-year-safety-and-efficacy-of-deucravacitinib-in-systemic-lupus-erythematosus-results-from-a-phase-2-program/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/four-year-safety-and-efficacy-of-deucravacitinib-in-systemic-lupus-erythematosus-results-from-a-phase-2-program/

.jpg)
.jpg)