Session Information
Session Type: Abstract Session
Session Time: 12:00PM-12:15PM
Background/Purpose: Biologic therapies for moderate to severe psoriasis (PsO) include inhibitors of IL-17 (IL-17i), IL-23i, IL-12/23i, and tumor necrosis factor (TNFi). Previous studies that assessed whether biologic treatments can prevent inflammatory arthritis (IA; including PsA) in patients with PsO have not accounted for potential confounders such as PsO severity, PsA/IA-related therapy, and physician preference. This study compared incident PsA/IA among patients with PsO who newly initiated IL-17i, IL-23i, IL-12/23i, or TNFi therapy.
Methods: Adults with ≥2 PsO diagnosis codes (International Classification of Disease [ICD] codes) ≥30 days apart who newly initiated an IL-17i, IL-23i, IL-12/23i, or TNFi between January 1, 2019, and April 17, 2024 (index date = first record), were included in this retrospective cohort analysis of electronic health records (EHR). Exclusion criteria included missing age/sex information, any diagnosis code for IA (PsA or other IA) any time before or within 14 days after the index date, or NSAID prescriptions in 12 months prior to (pre-index period) or on the index date to mitigate protopathic bias. Patient demographics, clinical characteristics, past treatments, and comorbidities in the pre-index period or at index were descriptively reported. We used the Kaplan-Meier (KM) method to estimate the cumulative incidence of PsA or other IA during the follow-up period, and a 2-sample test with Greenwood plug-in estimator for variance to compare the area under the curve (AUC; ie, restricted mean time lost [RMTL]) between cohorts up to the last common time point. Propensity score matching (PSM) was used to attempt to balance covariates for each pairwise comparison. Covariates included key disease and demographic characteristics (including but not limited to those in Table 1) from both structured and natural language processing–derived EHR data.
Results: Before PSM, the most common biologics by class were secukinumab (IL-17i), risankizumab (IL-23i), ustekinumab (IL-12/23i), and adalimumab (TNFi; Table 2). After PSM, 1444 patients were included in the IL-17i vs IL-23i cohort, 765 patients were in the IL-17i vs IL-12/23i cohort, and 1476 patients were in the IL-17i vs TNFi cohort (Table 1). Patient demographics, past treatment, clinical characteristics, and mean times from PsO onset to index and follow-up were balanced across cohorts. KM analysis revealed a trend toward lower RMTL for time with PsA/IA through 57 months in patients treated with IL-17i vs IL-23i (P=.052; Figure 1). RMTL for time with PsA/IA through 57 months was significantly lower in patients treated with IL-17i vs IL-12/23i (P< .05) and in those treated with IL-17i vs TNFi through 58 months(P< .01). On average, patients treated with IL-17i had a 45% reduction in time with PsA/IA vs those treated with IL-23i, a 61% reduction in time with PsA/IA vs those treated with IL-12/23i, and a 74% reduction in time with PsA/IA vs those treated with TNFi over the available follow-up.
Conclusion: In this retrospective cohort analysis of EHR data employing PSM, patients with PsO treated with IL-17i had lower incidence of PsA/IA development through 5 years post-treatment initiation than patients treated with IL-23i, IL-12/23i, or TNFi therapies.
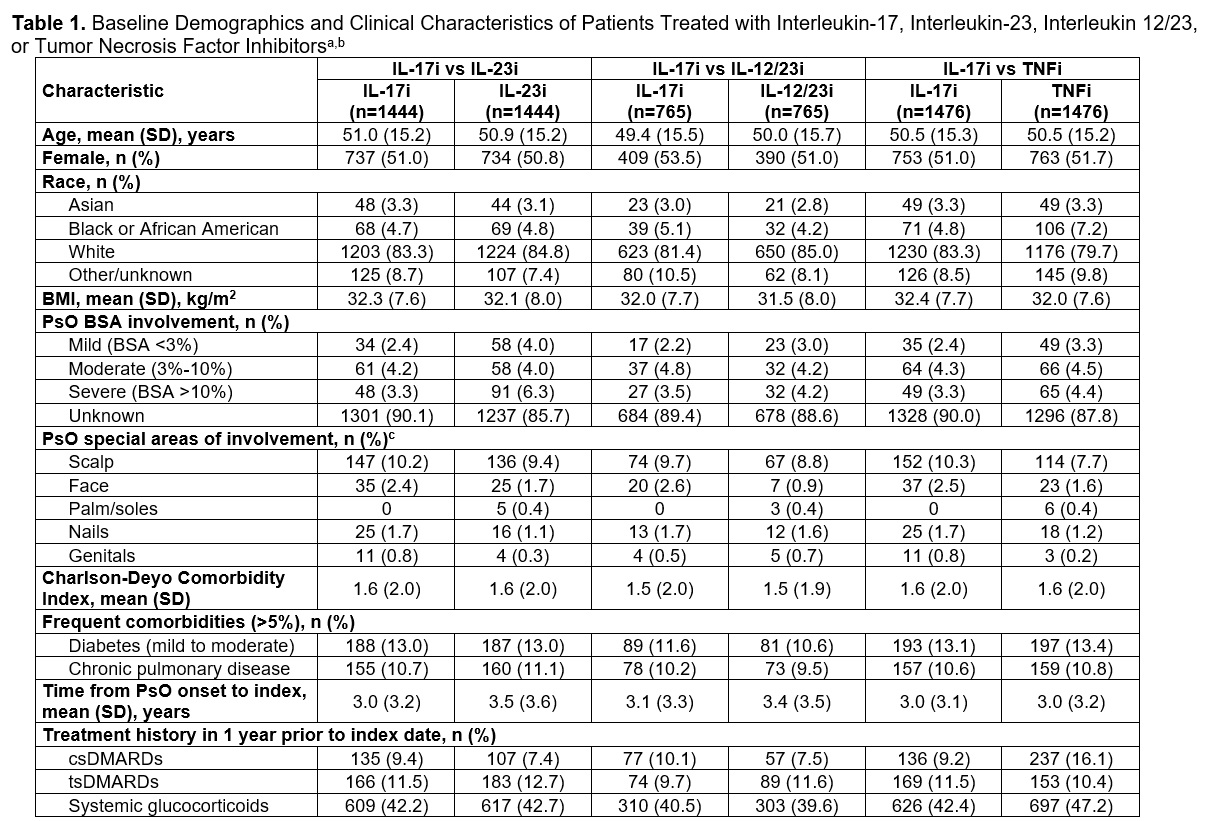 BMI, body mass index; BSA, body surface area; csDMARD, conventional synthetic disease-modifying anti-rheumatic drug; IL-12/23i, interleukin 12/23 inhibitor; IL-17i, interleukin 17 inhibitor; IL-23i, interleukin 23 inhibitor; PsO, psoriasis; TNFi, tumor necrosis factor inhibitor; tsDMARD, targeted synthetic disease-modifying anti-rheumatic drug.
BMI, body mass index; BSA, body surface area; csDMARD, conventional synthetic disease-modifying anti-rheumatic drug; IL-12/23i, interleukin 12/23 inhibitor; IL-17i, interleukin 17 inhibitor; IL-23i, interleukin 23 inhibitor; PsO, psoriasis; TNFi, tumor necrosis factor inhibitor; tsDMARD, targeted synthetic disease-modifying anti-rheumatic drug.
a Propensity score–matched results.
b For each pairwise comparison, standardized mean differences were < 0.2 for all patient characteristics.
c During 12-month pre-index period. Data are not mutually exclusive.
.jpg) IL-12/23i, interleukin 12/23 inhibitor; IL-17i, interleukin 17 inhibitor; IL-23i, interleukin 23 inhibitor; TNFi, tumor necrosis factor inhibitor.
IL-12/23i, interleukin 12/23 inhibitor; IL-17i, interleukin 17 inhibitor; IL-23i, interleukin 23 inhibitor; TNFi, tumor necrosis factor inhibitor.
a Before propensity score matching.
.jpg) AUC, area under the curve; IA, inflammatory arthritis; ICD, International Classification of Diseases; IL-12/23i, interleukin 12/23 inhibitor; IL-17i, interleukin 17 inhibitor; IL-23i, interleukin 23 inhibitor; PsA, psoriatic arthritis; PsO, psoriasis; TNFi, tumor necrosis factor inhibitor.
AUC, area under the curve; IA, inflammatory arthritis; ICD, International Classification of Diseases; IL-12/23i, interleukin 12/23 inhibitor; IL-17i, interleukin 17 inhibitor; IL-23i, interleukin 23 inhibitor; PsA, psoriatic arthritis; PsO, psoriasis; TNFi, tumor necrosis factor inhibitor.
a ICD diagnostic codes for PsA included ICD-9-CM: 696.0 and ICD-10-CM: L40.5x; ICD diagnostic codes for other IA included ICD-9-CM: 718.5, 716.85, 714.9, 714.0, and 720x, and ICD-10-CM: M06.4, M46.1, M45x, M46.9x, M07.6x, and M05x.
b Propensity score–matched results.
To cite this abstract in AMA style:
Armstrong A, Merola J, Wang G, Faucher A, Taiji R, Vekeman F, Shew A, Nguyen T, Coates L. Comparison of Incidence of Psoriatic Arthritis in Patients With Psoriasis Treated With Interleukin-17 Inhibitors vs Interleukin-23 Inhibitors, Interleukin-12/23 Inhibitors, and Tumor Necrosis Factor Inhibitors in Real-World Practice: A Retrospective Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparison-of-incidence-of-psoriatic-arthritis-in-patients-with-psoriasis-treated-with-interleukin-17-inhibitors-vs-interleukin-23-inhibitors-interleukin-12-23-inhibitors-and-tumor-necrosis-factor-i/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-incidence-of-psoriatic-arthritis-in-patients-with-psoriasis-treated-with-interleukin-17-inhibitors-vs-interleukin-23-inhibitors-interleukin-12-23-inhibitors-and-tumor-necrosis-factor-i/
