Session Information
Session Type: Abstract Session
Session Time: 12:45PM-1:00PM
Background/Purpose: RA-ILD is associated with high mortality, but the optimal treatment approach is unclear. While immunosuppressants may treat inflammatory components of ILD, there are concerns about pro-fibrotic effects and increased infection risk. Prior studies investigating specific biologic or targeted synthetic DMARDs (b/tsDMARDs) in RA-ILD had small sample size, and no randomized trials have been performed. Despite this limited evidence, the 2023 ACR/CHEST guideline conditionally recommended rituximab as initial RA-ILD therapy.
Methods: Nationwide data from Medicare Parts A/B/D from 1/1/2006 to 12/31/2019 (n=7,993,101 patients) served as the data source. We identified patients with RA-ILD to emulate target trials comparing b/tsDMARDs. RA-ILD patients had ≥2 RA ICD-9/10 codes, ≥1 DMARD prescription 7-365 days after RA ICD code, and ≥2 ILD ICD codes, including ≥1 ILD ICD code from a pulmonologist (PPV 81%). We emulated target trials comparing patients with RA-ILD initiating abatacept, tocilizumab, JAK inhibitors, or TNF inhibitors, each compared to rituximab. The index date was first b/tsDMARD after RA-ILD diagnosis. The primary outcome was a composite of respiratory hospitalization (including respiratory infections), lung transplant, or death. We constructed propensity scores (PS) for treatment selection using 46 pre-exposure variables including year of enrollment, demographic factors, RA-related factors (including line of therapy and oral glucocorticoids), pulmonary factors, comorbidities, medications, healthcare utilization, and Medicare low-income subsidy eligibility. After 1:1 PS matching and using an intention-to-treat follow up, we constructed Kaplan-Meier curves and used Cox regression to estimate hazard ratios for the composite outcome and its components.
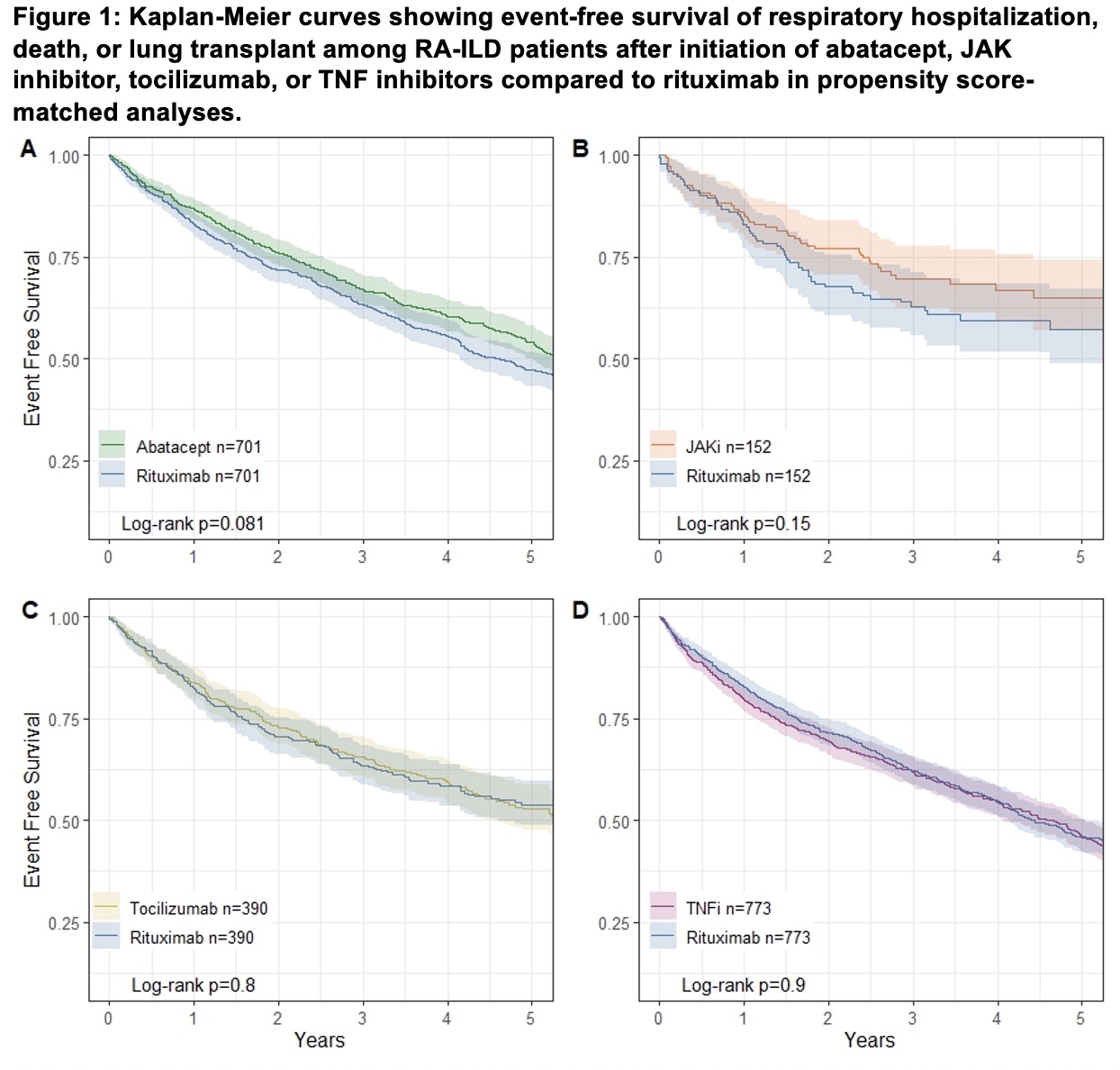
Results: After PS matching, we analyzed RA-ILD patients (mean age 74 years, 70% female) initiating abatacept (n=701), JAK inhibitor (n=152), tocilizumab (n=390), or TNFi (n=773), each compared to an equal number of rituximab initiators. Separate emulated target trials were used for each two-way comparison. PS matching successfully achieved balance in baseline covariates between treatment groups. Comparing each b/tsDMARD to rituximab, there were no significant differences in the risk of the composite outcome of respiratory hospitalization, lung transplant, or death: abatacept (HR 0.88 95%CI 0.76-1.02), JAK inhibitor (HR 0.75 95%CI 0.51-1.11), tocilizumab (HR 1.03 95%CI 0.84-1.28), or TNF inhibitor (HR 1.01 95%CI 0.88-1.16), each compared to rituximab (Figure 1). Separate analyses found no statistically significant differences between b/tsDMARDs and rituximab for respiratory hospitalization or death (Figure 2).
Conclusion: Despite rituximab being conditionally recommended as first line treatment for RA-ILD in the recent ACR/CHEST guideline, we found no significant differences between rituximab and other b/tsDMARDs in the risk of respiratory hospitalization, lung transplant, or death. There were nonsignificant findings of decreased mortality in RA-ILD patients treated with abatacept or JAK inhibitors compared to rituximab. These findings highlight the need for randomized controlled trials in RA-ILD.
To cite this abstract in AMA style:
McDermott G, Solomon D, Sparks J, Desai R. Target trial emulation of biologic and targeted synthetic disease-modifying antirheumatic drugs in rheumatoid arthritis-associated interstitial lung disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/target-trial-emulation-of-biologic-and-targeted-synthetic-disease-modifying-antirheumatic-drugs-in-rheumatoid-arthritis-associated-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/target-trial-emulation-of-biologic-and-targeted-synthetic-disease-modifying-antirheumatic-drugs-in-rheumatoid-arthritis-associated-interstitial-lung-disease/

.jpg)