Session Information
Date: Wednesday, October 29, 2025
Title: Abstracts: Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes III (2645–2650)
Session Type: Abstract Session
Session Time: 10:45AM-11:00AM
Background/Purpose: Disease activity measures in SLE have important limitations that restrict their performance in clinical practice and research. The Lupus Foundation of America-Rapid Evaluation of Activity in Lupus (LFA-REAL) is a novel and simple SLE disease activity instrument. Each organ and active symptom/sign receives a unique visual analog score (0-100mm each), allowing sensitive detection and granular understanding of disease fluctuations. The system allows rapid yet versatile evaluation of progress at the level of symptoms, organs, or overall disease, and addresses pitfalls in glossary defined cutoffs for scoring that limit the utility of the SLE Disease Activity Index (SLEDAI) and British Isles Lupus Assessment Group Index (BILAG). Here, we evaluate the sensitivity of the LFA-REAL to detect changes in the clinician’s global Impression of clinically significant change (CGIC) compared to the SLEDAI and BILAG.
Methods: An observational, longitudinal, prospective study of SLE patients was conducted at four lupus centers. Each study participant was evaluated at a baseline and follow-up visit. The same clinician scored the SLEDAI, BILAG, and LFA-REAL. At the follow-up visit, the physician also rated the CGIC, which rates no change vs clinically significant change compared to the previous visit. Agreement between changes in disease activity scores and CGIC was analyzed, generating ROC curves to assess the accuracy of each instrument to detect the clinician’s overall conclusion about the degree of change.
Results: Of 163 patients enrolled, 145 completed follow-up visits, 124 (85%) were women, mean age was 41.4 +/- 12.9 years (Table 1). Among the 145 patients with follow-up, 33 (23%) had CGIC clinically significant disease improvement, 25 (17%) worsening, and 87 (60%) no change. For the CGIC improvement group the median change in LFA-REAL from visit 1 to visit 2 (V2-V1) (ΔLFA-REAL) was -36 (IQR: -62 ; -20), for the group with no change in CGIC the median change in LFA-REAL was 0 (IQR: -20 ; 10), and for those who worsened: 27 (6 ; 34) (Figure 1). Compared to BILAG and SLEDAI, LFA-REAL demonstrated the greatest sensitivity for clinical change in either direction, with area under the curve (AUC) of 0.8385 for improvement and 0.8305 for worsening. BILAG AUCs were 0.7461 and 0.7553, and SLEDAI AUCs were 0.7385 and 0.7362, respectively (Figure 2). Using DeLong’s test, the AUC for LFA-REAL was significantly greater than that of SLEDAI for detecting improvement (p = 0.042) and trended towards greater sensitivity than BILAG (p = 0.073). Detection of worsening was similar compared to SLEDAI (p = 0.093) and BILAG (p = 0.188). Of the 25 patients classified as having clinical worsening by CGIC, 8 (32%) required a change in treatment. LFA-REAL identified all 8 of these patients as having clinically significant worsening, compared to 4 (50%) detected by BILAG and 2 (25%) by SLEDAI.
Conclusion: LFA-REAL demonstrated greater accuracy than SLEDAI and BILAG in detecting clinically significant disease changes in SLE. These findings support the potential utility of this easy to score instrument for monitoring disease activity in both clinical practice and research.
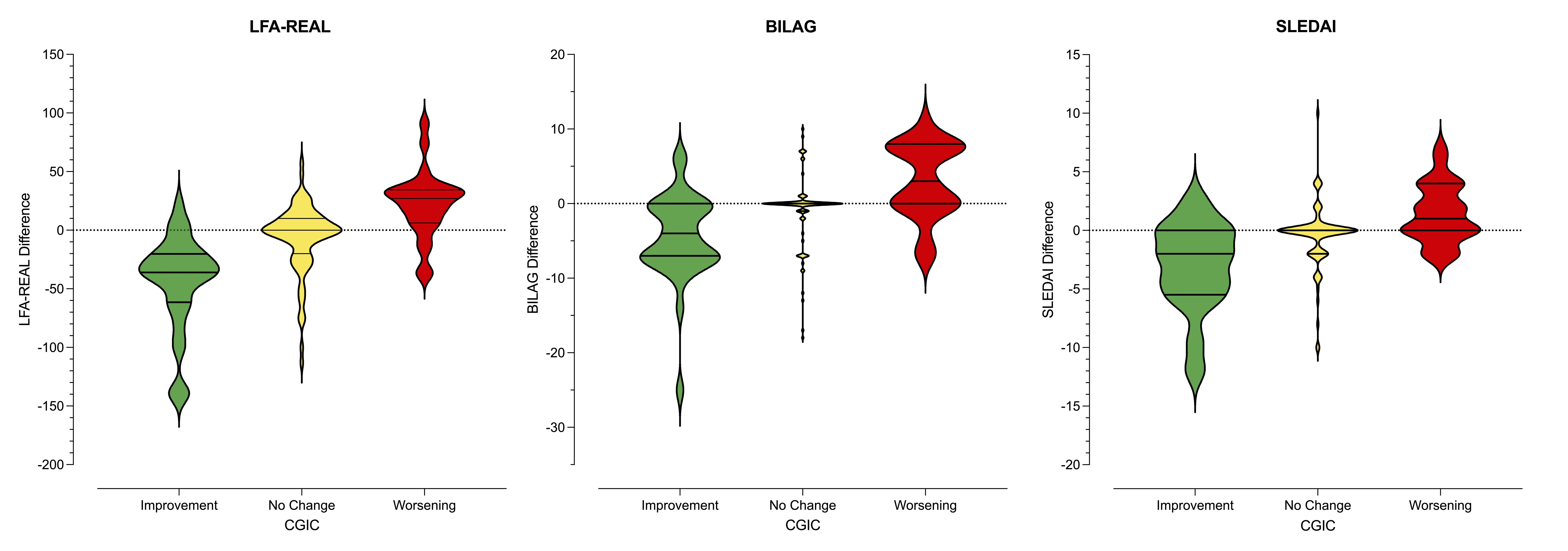 Table 1. Baseline demographic and clinical characteristics of the participants.
Table 1. Baseline demographic and clinical characteristics of the participants.
.jpg) Figure 1. Change in disease activity scores according to CGIC group
Figure 1. Change in disease activity scores according to CGIC group
.jpg) Figure 2. ROC curves: Ability of disease activity scores to identify clinical improvement or worsening according to CGIC
Figure 2. ROC curves: Ability of disease activity scores to identify clinical improvement or worsening according to CGIC
To cite this abstract in AMA style:
Nordmann-Gomes A, Khalili L, Aranow C, mackay M, Kim M, Kamen D, Arriens C, Souvignier M, Tang W, Suh S, Dall'Era M, Merrill J, Askanase A. LFA-REAL Outperforms SLEDAI and BILAG in Detecting Clinical Change in Lupus Activity [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/lfa-real-outperforms-sledai-and-bilag-in-detecting-clinical-change-in-lupus-activity/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lfa-real-outperforms-sledai-and-bilag-in-detecting-clinical-change-in-lupus-activity/
