Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: TNF-a blocker is the only treatment that shows a significant effect on spinal inflammation and life quality of patients with ankylosing spondylitis (AS). However, the predicting factors for the metrology outcome in AS patients treated with TNF-a blockers have not been reported. In this study, we investigated whether the early clinical efficacy of TNF-a blockers determines the metrology outcome in AS patients.
Methods: A retrospective study was conducted in a total of 119 cases who were treated with TNF-a blockers for 21 months (85 patients with one TNF-a blocker and 17 patients with two). Patients were evaluated at baseline, after three months of TNF-a blockers, and then every six months. Clinical efficacy was evaluated using Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), ASAS20, ASAS40, ASAS5/6, BASDAI50, and acute phase reactants (ESR and CRP). Metrology outcome was assessed by BASMI and chest expansion. Primary resistance was defined as a failure of improvement of at least 20% or of absolute improvement at least two units in BASDAI scores at month 3. Kaplan-Meier (KM) survival curves were plotted to determine the rates of continuation of TNF-a blockers (drug survival).
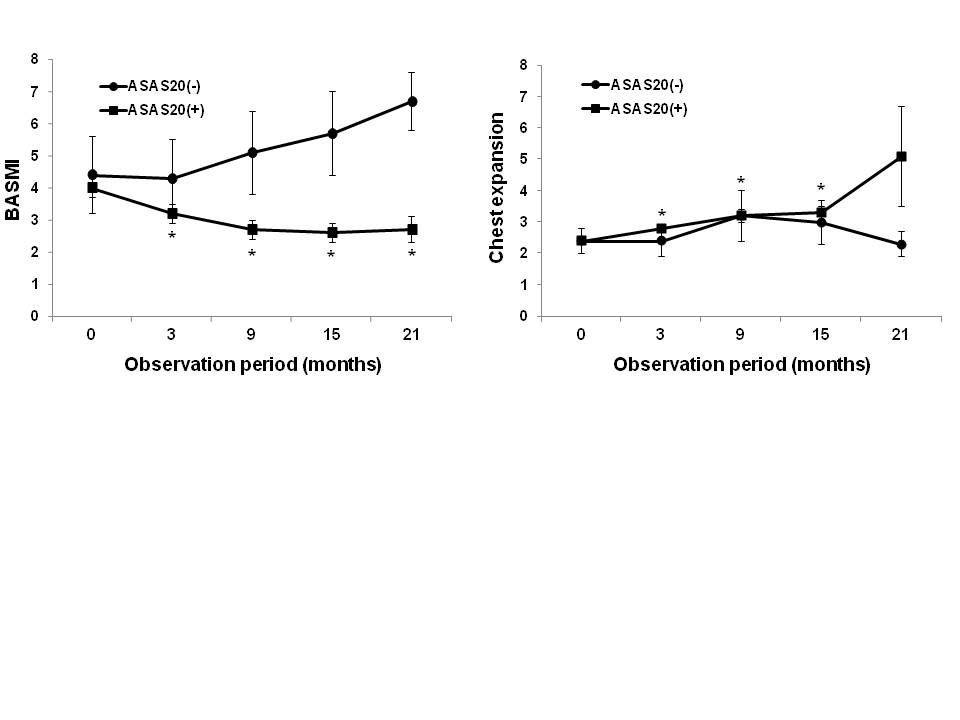
Results: Etanercept was used in 57 cases (47.9%), adalimumab in 49 (41.2%), and infliximab in 13 (10.3%). TNF-a blockers showed a similar drug survival rate and clinical efficacy. Primary resistance developed in 5 patients (4.2%). ASAS20 response rate was 90.2%, 91.7%, 92.4%, and 90% at months 3, 9, 15, and 21, respectively. TNF-a blockers showed a clinical response rate ranging 77.2 to 83.5% by ASAS40, 74.6 to 84.5% by ASAS5/6, and 74.3 to 92.1% by BASDAI50. TNF-a blockers were associated with significantly improved metrology indices including BASMI, BASMI components, and chest expansion at months 3, 9, 15, and 21. The changes in the BASMI significantly correlated with the changes in the BASDAI (r=0.260, P < 0.001), BASFI (r=0.726, P < 0.001), patient's global assessment score (r=0.479, P < 0.001), physician's global assessment score (r=0.517, P < 0.001), pain score (r=0.394, P < 0.001), and acute phase reactants (ESR, r=0.184, P < 0.001; CRP, r=0.118, P < 0.001). ASAS20 responders at month 3 had a significant reduction in BASMI and chest expansion, compared to those of baseline (p<0.001), while ASAS20 non-responders did not show a significant change. Reduction of metrology indices was maintained until month 21.
Conclusion: ASAS20 response at month 3 may be a valuable predictor for metrology outcome. Further studies are required to determine whether ASAS20 at month 3 is a good predictor of radiologic results along with metrology outcome in assessment of axial involvement.
Disclosure:
E. J. Nam,
None;
J. S. Eun,
None;
N. R. Kim,
None;
J. W. Kang,
None;
C. H. Im,
None;
Y. M. Kang,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-early-clinical-response-of-tnf-alpha-blockers-is-a-predictor-of-metrology-outcome-in-ankylosing-spondylitis/