Session Information
Session Type: Abstract Session
Session Time: 1:45PM-2:00PM
Background/Purpose: The MARCH study evaluates the efficacy of methotrexate monotherapy in patients with chikungunya-associated arthritis and aims to elucidate its pathologic mechanism via cytokine analysis. As of May 05, 2025, 140 of the targeted 162 participants have been enrolled, with recruitment ongoing.
Methods: Enrollment began on July 18, 2023, in Barranquilla, Colombia. This randomized, placebo-controlled, double-blind, parallel-design Phase 3 clinical trial investigates six months of methotrexate/placebo randomized 2:1 in adult volunteers with a history of Chikungunya Arthritis. An optional dose escalation from 15 mg/week to 20 mg/week is permitted at six weeks, followed by an optional open-label extension from six to 12 months, with follow-up until 24 months. Immunological profiling was performed using a Meso Scale Discovery (MSD) multiplex immunoassay platform, which enables the simultaneous, high-sensitivity quantification of multiple cytokines and chemokines in serum samples.
Results: Of 245 screened participants, 140 were enrolled (111 females, 29 males), and 132 remain actively in treatment (Figure 1). The mean age of the cohort is 55.54 +/- 12.93 years, with 53% having completed elementary education. Over six months, there was a significant reduction (p < 0.001) in disease severity, as measured by arthritis disease activity by the disease activity score-28 (DAS-28), disability by the health assessment questionnaire (HAQ), and arthritis flare activity by the flare score, indicating a shift from moderate/severe to mild disease activity, improved functional capacity, and less flare intensity (Figure 2). Immunological analysis revealed statistically significant increases in wound healing cytokines including Eotaxin, B-NGF, TGFB-2, TGFB-3, and HGF, while inflammatory and pain cytokines such as RANKL/TNFSF11, GM-CSF, IL-10, IL-17, IL-1B, IL-4, IL-6, IL-8, and Substance P showed significant decreases over time. TNF-α, TGFB-1, and IL-2 exhibited slight, non-significant increases, whereas IFN-γ demonstrated a non-significant decrease (Figure 3).
Conclusion: Interim analysis indicates that methotrexate is generally well-tolerated in treating chikungunya-related arthritis. The study’s cohort, with a mean arthritis duration post-infection of approximately ten years, provides valuable insights into the chronic impact of the disease. The evolving immunological profile suggests transitioning from an acute pro-inflammatory state to a reparative state. These findings underscore the complex interplay between inflammatory and regulatory mechanisms in chronic alphaviral arthritis and are expected to inform future treatment guidelines. The study remains blinded, with most enrolled participants having completed the closed-label phase and currently in the open-label follow-up stage. As the study is still blinded, it remains unclear whether the observed clinical and immunological changes are attributable to the therapeutic effect of methotrexate or reflect the natural course of disease improvement.
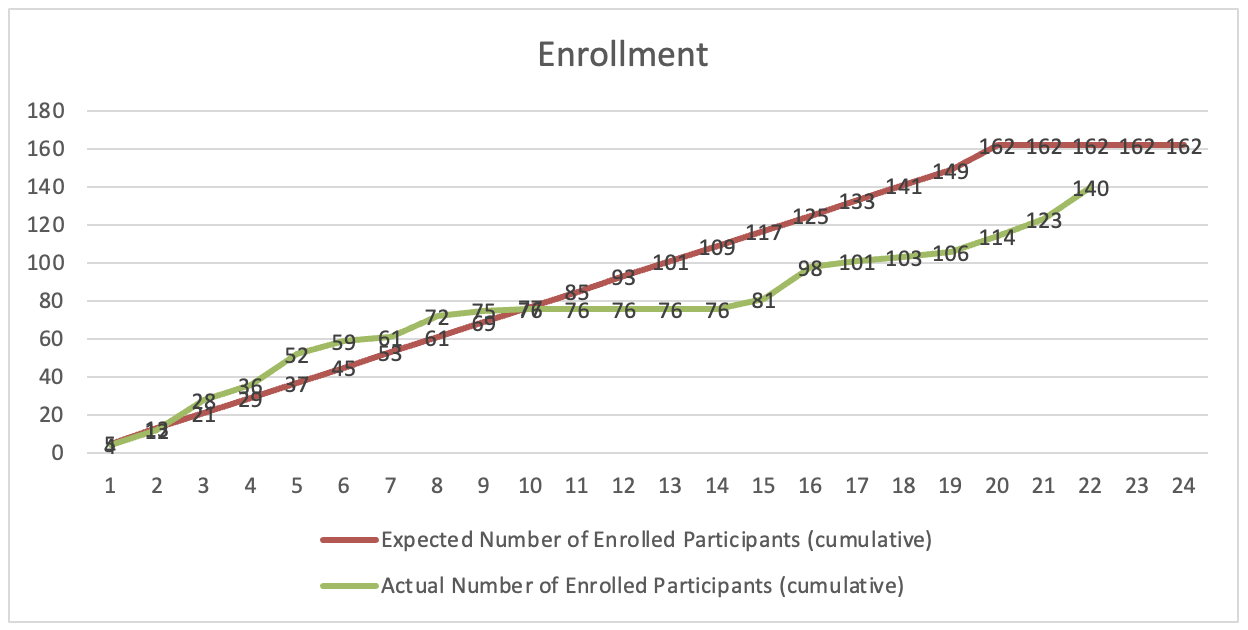 Figure 1. Cumulative enrollment of participants over time in the MARCH trial. The green line represents the actual number of participants enrolled each month, while the red line indicates the expected cumulative enrollment target. As of Month 22, 140 participants have been enrolled out of the planned 162, with final enrollment expected to be completed by June.
Figure 1. Cumulative enrollment of participants over time in the MARCH trial. The green line represents the actual number of participants enrolled each month, while the red line indicates the expected cumulative enrollment target. As of Month 22, 140 participants have been enrolled out of the planned 162, with final enrollment expected to be completed by June.
.jpg) Figure 2. Patient reported outcomes. 2a) Arthritis disease activity measured by the Disease Activity Score-28 2b) Disability measured by the Health Assessment Questionnaire 2c) Arthritis Flare Intensity measured by the Arthritis Flare Intensity Score adapted for use in Chikungunya arthritis.
Figure 2. Patient reported outcomes. 2a) Arthritis disease activity measured by the Disease Activity Score-28 2b) Disability measured by the Health Assessment Questionnaire 2c) Arthritis Flare Intensity measured by the Arthritis Flare Intensity Score adapted for use in Chikungunya arthritis.
.jpg) Figure 3. Cytokine measurement comparison between enrollment vs. 6 months after treatment with MTX/Placebo.
Figure 3. Cytokine measurement comparison between enrollment vs. 6 months after treatment with MTX/Placebo.
To cite this abstract in AMA style:
Sucerquia A, Forero J, Alzate J, Poon A, Firestein G, Moreland L, Cadena A, Lara J, pizarro O, Godoy J, Encinales L, Boyle D, Mendoza E, Herrera C, Chang A. Methotrexate Treatment of Arthritis Caused by Chikungunya Virus (MARCH) Study: Anti-inflammatory cytokine signals related to decreased arthritis disease activity over time [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/methotrexate-treatment-of-arthritis-caused-by-chikungunya-virus-march-study-anti-inflammatory-cytokine-signals-related-to-decreased-arthritis-disease-activity-over-time/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-treatment-of-arthritis-caused-by-chikungunya-virus-march-study-anti-inflammatory-cytokine-signals-related-to-decreased-arthritis-disease-activity-over-time/
