Session Information
Session Type: Abstract Session
Session Time: 2:00PM-2:15PM
Background/Purpose: Methotrexate laboratory monitoring is highly resource intensive, and recent evidence questions whether the true toxicity of methotrexate has been over-estimated. Therefore, we aimed to determine the incidence of clinically significant methotrexate toxicity.
Methods: We leveraged a population-based cohort in a geographically defined area. We screened these 1.4 million individuals for adults with ≥2 diagnostic codes for systemic rheumatic disease and new initiation of low-dose (≤25 mg/week) methotrexate between January 1, 2012 and December 31, 2024. We manually confirmed incident methotrexate use, recorded covariates including frequency of methotrexate laboratory monitoring as compared to ACR guidelines (≥3 in the first 3 months, ≥3 in the next 3-12 months, and at least every 3 months thereafter), determined whether each potential methotrexate-related abnormality [AST (high), ALT (high), creatinine (high), hemoglobin (low), platelets (low), or white blood cells (low)] was clinically significant (defined as temporary or permanent methotrexate dose reduction and/or discontinuation), and quantified healthcare resources used as a result of any abnormalities. We calculated the incidence of clinically significant methotrexate toxicity per 100 person-years.
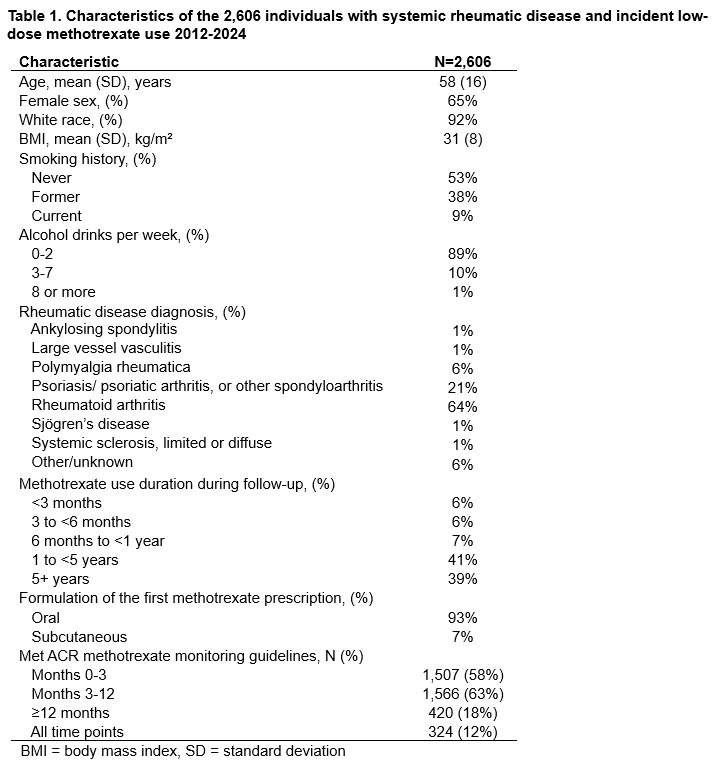
Results: Of the 3,558 individuals reviewed, we included all 2,606 who met the above inclusion criteria (mean age 58 years, 65% female, mean 4.3 [SD 3.1] years methotrexate use spanning 11,169 total person-years, 324 [12%] meeting ACR monitoring guidelines across all time points, Table 1). Many patients developed laboratory abnormalities (Table 2). However, only 97 of 4,847 (2.0%) of these abnormalities were clinically significant (0.9 per 100 person-years). Of those, 31 (32%) resulting in a reduced dose of methotrexate, and 66 (68%) resulting in permanent discontinuation, mostly for elevated AST and/or ALT (Table 2, Figure 1). On the other hand, the methotrexate monitoring abnormalities led to an additional 4,546 blood draws, 84 healthcare visits, 104 imaging studies, 16 bone marrow biopsies, and 2 liver biopsies.
Conclusion: This first population-based study of manually-validated methotrexate toxicity events found that the incidence of clinically significant methotrexate toxicity was low, and healthcare resource utilization in response to abnormal labs was high. These results suggest that less frequent methotrexate monitoring may be justified. Future work will elucidate the populations that most benefit from less frequent monitoring and identify the optimal monitoring protocols.
To cite this abstract in AMA style:
Reed G, Yang J, El Hasbani G, Crowson C, Langenfeld H, Sparks J, England B, Schmajuk g, Michaud K, Davis J, Kronzer V. Rare Clinically Significant Methotrexate Toxicity Despite Frequent Laboratory Abnormalities: A Population-Based Study of Methotrexate Monitoring [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/rare-clinically-significant-methotrexate-toxicity-despite-frequent-laboratory-abnormalities-a-population-based-study-of-methotrexate-monitoring/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rare-clinically-significant-methotrexate-toxicity-despite-frequent-laboratory-abnormalities-a-population-based-study-of-methotrexate-monitoring/

.jpg)
.jpg)