Session Information
Date: Tuesday, October 28, 2025
Title: (2524–2546) Vasculitis – Non-ANCA-Associated & Related Disorders Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: IgG4-related disease (IgG4-RD) is a chronic immune-mediated fibroinflammatory condition characterized by fibrosis and organ damage. Although IgG4-RD is increasingly recognized, real-world clinical burden remains poorly understood. This study aims to characterize the US IgG4-RD population, their diagnostic journey, clinical characteristics, treatment patterns, and burden of illness.
Methods: Data were derived from the Adelphi IgG4-RD Disease Specific Programme (DSP)™, a cross-sectional survey of patients with IgG4-RD and their providers in the United States, collected from 2023 to 2024. Physicians provided data on patient demographics, symptomology, treatment and clinical outcomes. Patient reported outcomes (PROs) including, Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue, EuroQol 5-dimension 5-level (EQ-5D-5L), and Work Productivity and Activity Impairment (WPAI) were collected from patients.
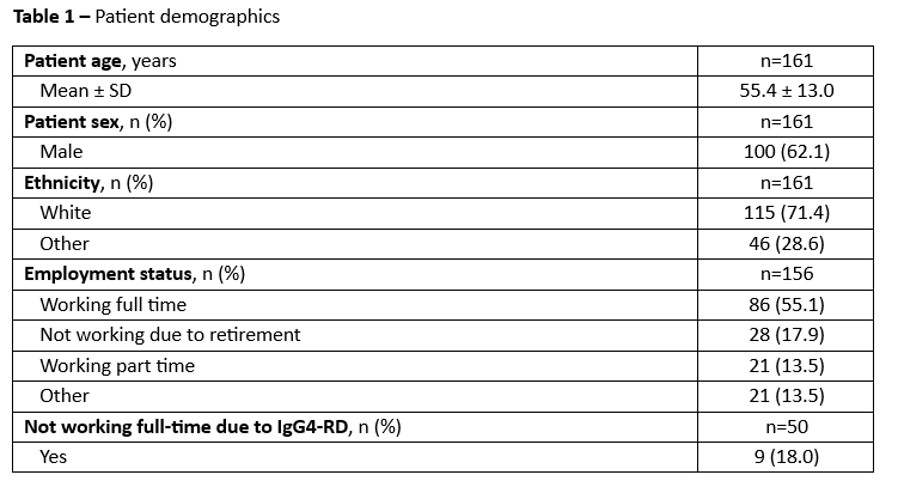
Results: In total, 41 physicians returned data on 161 patients, and 27 patients completed surveys. Mean patient age was 55.4 ± 13.0 years, 62.1% were male and 71.4% were White (Table 1). Mean age at symptom onset was 50.1 ± 13.2 years and time to diagnosis was 11.5 ± 21.8 months. Fatigue (41.6%), joint pain (23.0%), and weight loss (22.4%) were the most commonly reported symptoms at first consultation. The pancreas (31.7%), parotid glands (22.4%) and retroperitoneal fibrosis (19.3%) were the most commonly reported organ manifestations (Table 2).Primary care physicians (47.4%), gastroenterologists (20.4%) and rheumatologists (11.2%) were most commonly consulted about initial symptoms. Patients required a mean of 3.3 ± 2.0 consultations and providers, on average, used 13.4 ± 7.8 assessments to aid diagnosis. A different diagnosis than IgG4-RD was initially suspected for 90.2% of patients, most commonly conditions such as pancreatitis (15.4%), chronic fatigue syndrome (14.7%) and vasculitis (14.0%).Treatment initiation was, on average, 8.9 ± 13.3 months following diagnosis. Rheumatologists (70.2%), gastroenterologists (41.0%) and primary care physicians (29.2%) primarily managed patients’ treatment regimens. Prednisone was the most common first line therapy (66.3%), followed by rituximab (16.9%) and azathioprine (10.6%).Hospitalizations due to IgG4-RD were reported for 14.3% of patients in the 12 months prior to the survey. Among those hospitalized, 68.4% were admitted via the emergency room. Patients reported impact to quality of life as characterized by PROs presented in Table 3.
Conclusion: Patients face delays in receiving a correct IgG4-RD diagnosis, with the diagnostic journey often involving multiple consultations and tests. IgG4-RD presents heterogeneously, and the majority of patients reported substantial burden of illness and impact to daily lives. Earlier clinical diagnosis and effective management may improve health outcomes and quality of life among patients with IgG4-RD.
To cite this abstract in AMA style:
Hernandez-Barco Y, Park J, Wani R, Meyers A, Baker M, Calabrese L, Patterson K, Connolly H, Birija S, Culver E. Diagnostic Journey, Clinical Burden, And Quality Of Life Of Patients With IGg4-Related Disease: Results Of A Cross-Sectional Survey Of Patients And Physicians In The United States [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/diagnostic-journey-clinical-burden-and-quality-of-life-of-patients-with-igg4-related-disease-results-of-a-cross-sectional-survey-of-patients-and-physicians-in-the-united-states/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/diagnostic-journey-clinical-burden-and-quality-of-life-of-patients-with-igg4-related-disease-results-of-a-cross-sectional-survey-of-patients-and-physicians-in-the-united-states/

.jpg)
.jpg)