Session Information
Date: Tuesday, October 28, 2025
Title: (2524–2546) Vasculitis – Non-ANCA-Associated & Related Disorders Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Chronic progressive neuro-Behçet’s disease (CPNB) is characterized by progressive deterioration leading to disability and death. It has been appreciated that methotrexate (MTX) has beneficial effects for CPNB. Moreover, recent studies have demonstrated that infliximab (IFX) is effective for patients who had inadequate responses to MTX. However, it is unclear which patients should be treated and how, especially on the initiation of treatment. Thus, clinical markers of the severity would be helpful for decisions on treatment strategies. We therefore tried to determine severity classification criteria for introduction of IFX in patients with CPNB.
Methods: Twenty-six patients with CPNB (19 males, 7 females, ages 47.7±15.6 years [mean±SD]), who had been collected in a multicenter study by the Japanese Research Committee from 2011 to 2013, were followed up for 6-216 months (median 93.0 months). The provisional severity levels were retrospectively determined according to treatment regimens and responses of the patients, such as – Group (Gr)1: no progression with MTX alone, Gr 2: no progression with combined MTX and IFX, Gr 3: progression even with combination of MTX and IFX. Cerebrospinal fluid (CSF) IL-6 levels and brain MRI findings before starting treatment, and progressions of neurobehavior manifestations after treatment were evaluated. The relationship between these parameters and provisional severity levels was examined retrospectively. Based on the results, new severity criteria were established.
Results: CSF IL-6 was significantly lower in Gr 1 than in combined Gr 2+ Gr 3 (p=0.0039), despite no significant difference among the 3 groups (Figure 1). ROC analysis disclosed that a cut-off of 98.5 pg/ml of CSF IL-6 discriminated between Gr 1 and combined Gr 2+ Gr 3 with 80% sensitivity and 70% specificity (AUC 0.87, p=0.005) (Figure 2). On the other hand, 3 patients among the 26 patients lacked brainstem atrophy on MRI, in whom no progression of symptoms was observed. Thus, the severity of CPNBD (stage 1-3) was formulated according to the presence of brainstem atrophy and the elevation CSF IL-6 over 99.0 pg/ml (Table). The higher stage tended to be associated with the progression in the 26 patients (p=0.0925).
Conclusion: The severity classification criteria for CPNB, developed based on the presence of brainstem atrophy and the elevation of CSF IL-6 (over 99.0 pg/ml), were considered useful in determining treatment strategy. Thus, MTX alone would be recommended in stage 1, whereas combination of MTX and IFX should be considered in stage 3. As for patients in stage 2, IFX should be considered for patients with persistent CSF IL-6 over 99.0 pg/ml despite increased doses of MTX.
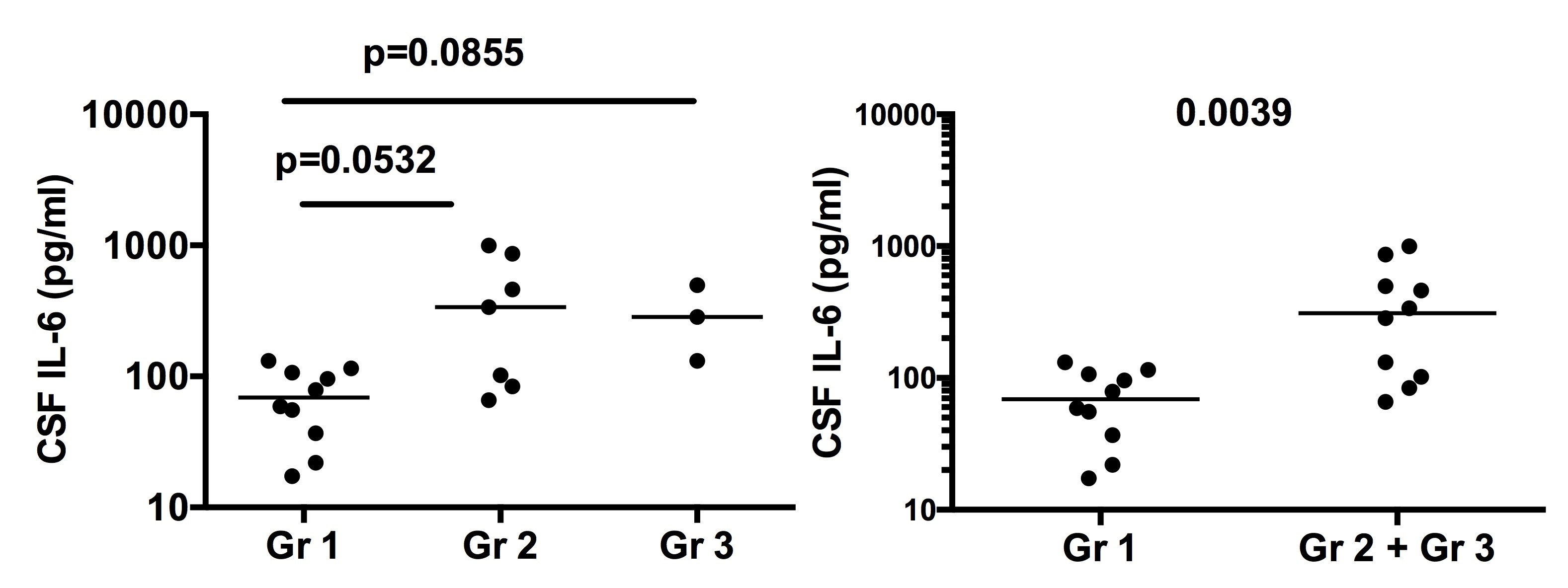 Figure 1 CSF IL-6 in 3 provisional severity groups.
Figure 1 CSF IL-6 in 3 provisional severity groups.
.jpg) Figure 2 ROC analysis for CSF IL-6 between Gr 1 and combined Gr 2 + Gr 3.
Figure 2 ROC analysis for CSF IL-6 between Gr 1 and combined Gr 2 + Gr 3.
.jpg) Table Suggested severity criteria for CPNBD
Table Suggested severity criteria for CPNBD
To cite this abstract in AMA style:
Hirohata S, Kikuchi H, Sawada T, Tono T, Takeno M, Kawachi I. Development of Severity Classification Criteria for Introduction of Infliximab for Chronic Pogressive Neuro-Behçet’s Disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/development-of-severity-classification-criteria-for-introduction-of-infliximab-for-chronic-pogressive-neuro-behcets-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-severity-classification-criteria-for-introduction-of-infliximab-for-chronic-pogressive-neuro-behcets-disease/
