Session Information
Date: Tuesday, October 28, 2025
Title: (2524–2546) Vasculitis – Non-ANCA-Associated & Related Disorders Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Worse health-related quality of life has been reported in individuals with polymyalgia rheumatica (PMR), yet the factors contributing to this burden – including the impact of glucocorticoids (GC) and GC-sparing agents – remain poorly understood. Longitudinal cohort studies incorporating patient-reported outcomes (PROs) can provide valuable insights to guide more patient-centered treatment. The objective of this study was to describe the baseline characteristics and PROs of patients in the ENRICH-PMR cohort, an ongoing longitudinal cohort of patients with PMR under rheumatology care.
Methods: A prospective cohort of patients with PMR was created within the Excellence Network in Rheumatology to Innovate Care and High-impact research (ENRICH), the largest US community-based, embedded, practice-based research network. Patients with a diagnosis of PMR routinely cared for in offices with access to a customizable in-office tablet app (I-PRO) deployed at routinely scheduled visits were invited to participate. The baseline I-PRO survey included information on date of diagnosis and current dose of GC use. Demographics, body mass index, and GC-sparing agent use was collected from electronic medical records. Comorbidities were identified using the RxRisk index, a validated medication-based measure of comorbidity. PROs collected quarterly included 3 PROMIS measures: physical function, pain interference, and fatigue; PROMIS sleep and depression were collected annually. RAPID-3 was used to assess disease activity, and data on Patient Acceptable Symptom State (PASS) was collected to assess patients’ perception of their health state. For this analysis, the most recent PROs available (i.e., closest to the time of enrollment) were included. Descriptive statistics were used to summarize the cohort and compare PROs by sex, using standardized mean differences (SMD) >0.10 to identify those that are likely clinically relevant.
Results: Between April and October 2024, 1,182 PMR patients completed the baseline survey. Most were female (64.8%), white (82.05), with mean (SD) age of 73.7 (9.1); nearly half were age ≥75. Over one-quarter (28.8%) were within two years of PMR diagnosis. The mean (SD) number of comorbidities was 7.8 (3.7). More than half (56.6%) were receiving GCs, and a majority (xx%) were on a GC-sparing agent, most commonly methotrexate.Compared to males, female patients had a worse RAPID-3. Similarly, female patients reported lower physical function (41.3 (9.2) vs 44.9 (9.3), respectively) and greater pain interference (56.4 (9.2) vs 53.3 (8.3), respectively) as measured by PROMIS scores. This difference was clinically relevant based on SMDs >0.10. Overall, 37.0% of the cohort reported their current health state, as affected by PMR, to not be acceptable.
Conclusion: This baseline data from a large, prospective cohort of patients with PMR under rheumatology care shows that PMR patients experience a substantial impact of their disease across multiple PRO domains, and more than one-third express dissatisfaction with their current symptom state. Ongoing analyses will examine factors associated with worse PROs to inform more personalized PMR management strategies.
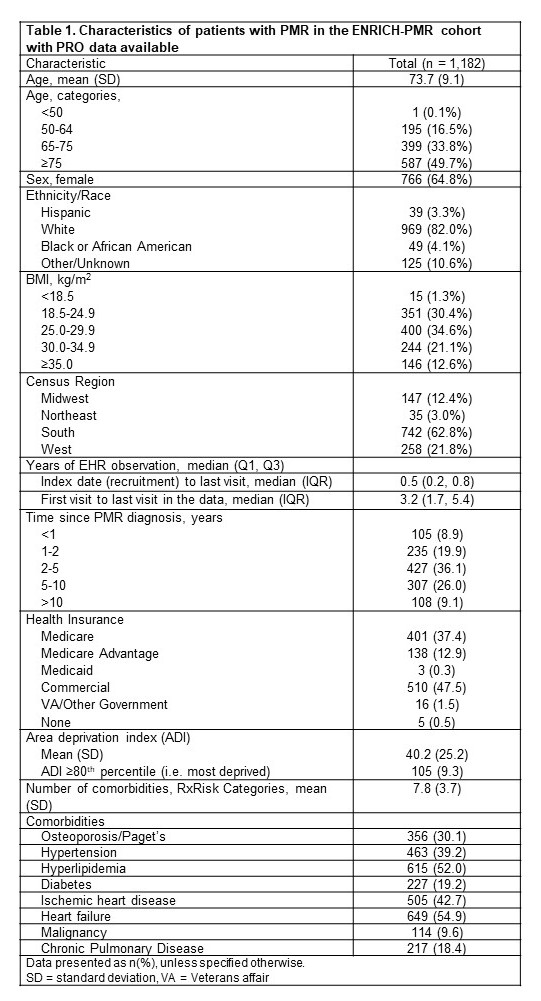 Table 1. Characteristics of patients with PMR in the ENRICH-PMR cohort with PRO data available
Table 1. Characteristics of patients with PMR in the ENRICH-PMR cohort with PRO data available
.jpg) Table 2. Current treatment in patients with PMR at time of inclusion in cohort
Table 2. Current treatment in patients with PMR at time of inclusion in cohort
.jpg) Table 3. Patient-reported outcomes by sex
Table 3. Patient-reported outcomes by sex
To cite this abstract in AMA style:
Sattui S, Mudano A, Su Y, Sodhi S, Xie F, Navarro-Millan I, Domsic R, Curtis J. Patient-reported outcomes in patients with polymyalgia rheumatica under rheumatology care: baseline data from the ENRICH-PMR cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/patient-reported-outcomes-in-patients-with-polymyalgia-rheumatica-under-rheumatology-care-baseline-data-from-the-enrich-pmr-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-reported-outcomes-in-patients-with-polymyalgia-rheumatica-under-rheumatology-care-baseline-data-from-the-enrich-pmr-cohort/
