Session Information
Date: Tuesday, October 28, 2025
Title: (2437–2469) Systemic Lupus Erythematosus – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Hydroxychloroquine (HCQ) plays an important role in the immunomodulation of Systemic Lupus Erythematosus (SLE), reducing the risk of flares and overall mortality. However, long-term HCQ use is associated with retinal toxicity, a common reason for discontinuation. The incidence, risk factors, and characteristics of SLE flares post HCQ discontinuation remain unclear. This study aims to identify markers that predict SLE flares in patients who discontinue HCQ.
Methods: Retrospective cohort of adult patients who met the 1997 American College of Rheumatology criteria for SLE and discontinued HCQ at a single center between 2012 and 2023. The index visit was the closest (within 3 months) to HCQ discontinuation, and the follow-up was 36 months from the index visit. Flares were assessed using the SLE Disease Activity Index (SLEDAI) flare rate instrument. We compared variables between patients who experienced flares and those who remained flare-free. Variables a priori identified as potential predictors were analyzed using a Cox regression model.
Results: 42 patients were included in this cohort. The mean age of patients was 50 years, 95% were female, 43% self-identified as White, 33% as Black. The mean duration of SLE was 15.7 years, with the mean HCQ dose before discontinuation being 4 mg/kg/day. The main reason for discontinuation was retinal toxicity (56%). At the index visit, 28% of patients had low complement levels, 39% were positive for anti-dsDNA, 55% were on immunosuppressive or biological treatment, and 26% were using prednisone at baseline (Table 1). A little over half of the patients flared during the follow-up period. In the first year after HCQ discontinuation, 12 patients (28.6%) flared, while 15 patients (35.7%) flared in the second year, and 14 patients (37.8%) flared in the third year. Five patients were lost to follow-up after 24 months. The comparison of baseline variables between the 21 patients who experienced at least one flare (of any severity) with 19 flare-free patients over the 36 months showed that patients with any flare had lower complement (50% vs. 7%, p = 0.011) and lower mean C3 levels (91.1 ± 20.1 vs. 124.7 ± 27.5 mg/dL, p = 0.001) at the index visit (Table 2). Kaplan-Meier curve showed that patients with low C3 levels flared more often and sooner than patients with normal C3 at baseline (log-rank p-value < 0.001). Univariate Cox regression analysis revealed that patients with low C3 levels at the index visit had a hazard ratio (HR) of 10.18 (95% CI: 3.26-31.84, p < 0.001) for any flare during follow-up, compared to patients with normal C3 levels (Figure 1).
Conclusion: In this cohort of 42 patients with SLE who discontinued HCQ, 21 patients (50%) experienced at least one flare over the 3 years. All patients with low C3 at baseline flared within the first 24 months, and low C3 was significantly linked to an increased risk of flares, especially in the first year. The use of immunosuppressants or glucocorticoids at the index visit was not associated with flare occurrence during follow-up. These findings suggest that monitoring C3 levels may help identify those at higher risk of complications after HCQ discontinuation. Further studies are needed to develop strategies for flare prevention in this setting.
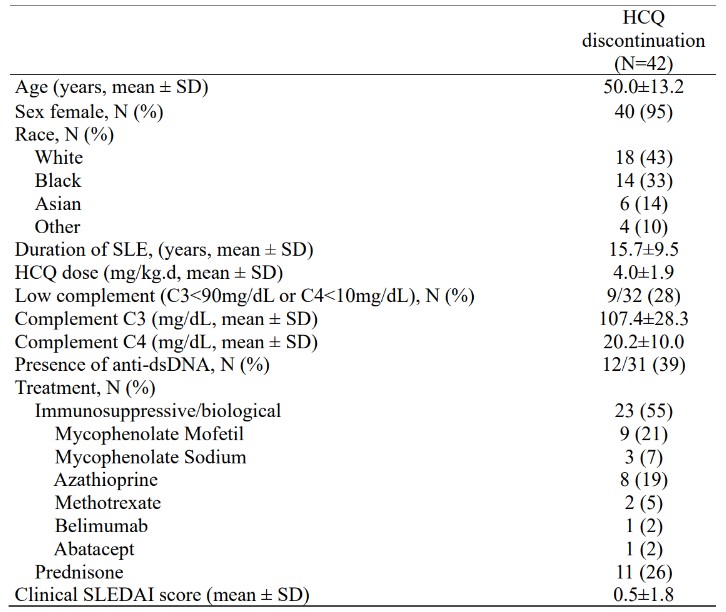 Table 1: Patient characteristics at the index visit. HCQ: hydroxychloroquine; SLE: Systemic Lupus Erythematosus; mg/kg.d: milligrams per kilogram per day; mg/dL: milligrams per deciliter; anti-dsDNA: double-stranded DNA antibody; SLEDAI: SLE Disease Activity Index.
Table 1: Patient characteristics at the index visit. HCQ: hydroxychloroquine; SLE: Systemic Lupus Erythematosus; mg/kg.d: milligrams per kilogram per day; mg/dL: milligrams per deciliter; anti-dsDNA: double-stranded DNA antibody; SLEDAI: SLE Disease Activity Index.
.jpg) Table 2. Comparison of baseline variables between patients with any flare over 3 years versus patients with no flare during follow-up. SLE: Systemic Lupus Erythematosus; HCQ: hydroxychloroquine; mg/kg.d: milligrams per kilogram per day; mg/dL: milligrams per deciliter; anti-dsDNA: double-stranded DNA antibody; SLEDAI: SLE Disease Activity Index.
Table 2. Comparison of baseline variables between patients with any flare over 3 years versus patients with no flare during follow-up. SLE: Systemic Lupus Erythematosus; HCQ: hydroxychloroquine; mg/kg.d: milligrams per kilogram per day; mg/dL: milligrams per deciliter; anti-dsDNA: double-stranded DNA antibody; SLEDAI: SLE Disease Activity Index.
.jpg) Figure 1: Kaplan-Meier survival analysis stratified by Complement C3 levels (log-rank p-value < 0.001) and Univariate Cox regression analysis with Hazard Ratio (HR).
Figure 1: Kaplan-Meier survival analysis stratified by Complement C3 levels (log-rank p-value < 0.001) and Univariate Cox regression analysis with Hazard Ratio (HR).
To cite this abstract in AMA style:
Delai M, Simon R, Mantovani Cardoso E, Kyttaris V. Hydroxychloroquine Discontinuation in Systemic Lupus Erythematosus: A Retrospective Cohort Study with 3-Year Follow-Up [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/hydroxychloroquine-discontinuation-in-systemic-lupus-erythematosus-a-retrospective-cohort-study-with-3-year-follow-up/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-discontinuation-in-systemic-lupus-erythematosus-a-retrospective-cohort-study-with-3-year-follow-up/
