Session Information
Date: Tuesday, October 28, 2025
Title: (2437–2469) Systemic Lupus Erythematosus – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The Definition Of Remission in SLE (DORIS) criteria were developed to align SLE remission definitions.1,2 Post hoc analyses of clinical trial data demonstrated that belimumab (BEL) plus standard therapy (ST) increases the proportion of patients with SLE in DORIS remission compared with placebo plus ST.3 As real-world SLE remission data for patients in the USA initiating BEL are scarce and there is a lack of clarity on characteristics of patients achieving remission, this retrospective, observational cohort study (GSK Study 222161) explored predictors of remission in this patient population.
Methods: Study period: Jan 1, 2013, to May 31, 2024. Data were derived from the OM1 PremiOM SLE dataset (OM1, Boston, MA, USA). Eligible patients had ≥1 BEL pharmacy/medical encounter during the identification period (Jan 1, 2014, to Oct 31, 2023). Real-world SLE remission was defined per DORIS proxy (SLEDAI/estimated SLEDAI score=0 [no disease activity]; Physician’s Global Assessment [PGA] < 2 on the numeric rating scale; prednisone equivalent dose ≤5 mg/day) and assessed up to 52 weeks post-BEL initiation. Kaplan–Meier estimates were used to assess the probability of achieving remission. Two censoring approaches assessed estimate robustness: 1) last available score – earlier of latest available SLEDAI or PGA score in the follow-up period and end of follow-up; 2) end of follow-up – earlier of 52 weeks post-index (first BEL receipt), last activity date, or date of data cutoff. Patient demographics and clinical characteristics associated with remission were identified in a prespecified exploratory analysis using a logistic regression model with remission as the dependent variable; odds ratio (OR), 95% confidence intervals (CI) and p-values were calculated.
Results: Of 398 patients, 94% were female and 71% White, with a mean (standard deviation) age of 51 (14) years at index.Unadjusted probability estimates showed patients aged ≥50 years (vs < 50 years), non-White patients (vs White) and those with no/low (vs moderate/high) disease activity had a slightly higher probability of achieving remission; however, estimates differed by censoring approach and confidence bounds overlapped (Figure 1).The adjusted model showed that older patients were significantly more likely to achieve remission by 52 weeks than younger patients (for each year of age OR: 1.03 [95% CI: 1, 1.06], p=0.0248; Figure 2). Patients of non-White race were significantly more likely to achieve remission than White patients (2.57 [1.27, 5.19], p=0.0092). Patients with moderate/high disease activity at baseline were significantly less likely to achieve remission than those with no/low disease activity (0.46 [0.21, 0.96], p=0.0378).
Conclusion: Predictors of real-world SLE remission with BEL include non-White race, older age, and no/low baseline disease activity. The association between minimal disease activity and higher remission rates supports the benefit of initiating belimumab treatment earlier in the course of disease.Funding: GSKReferences1van Vollenhoven R et al. Ann Rheum Dis 2017;76:554–612van Vollenhoven RF et al. Lupus Sci Med 2021;8:e0005383Parodis I et al. Lancet Rheumatol 2024;6(11):e751–61
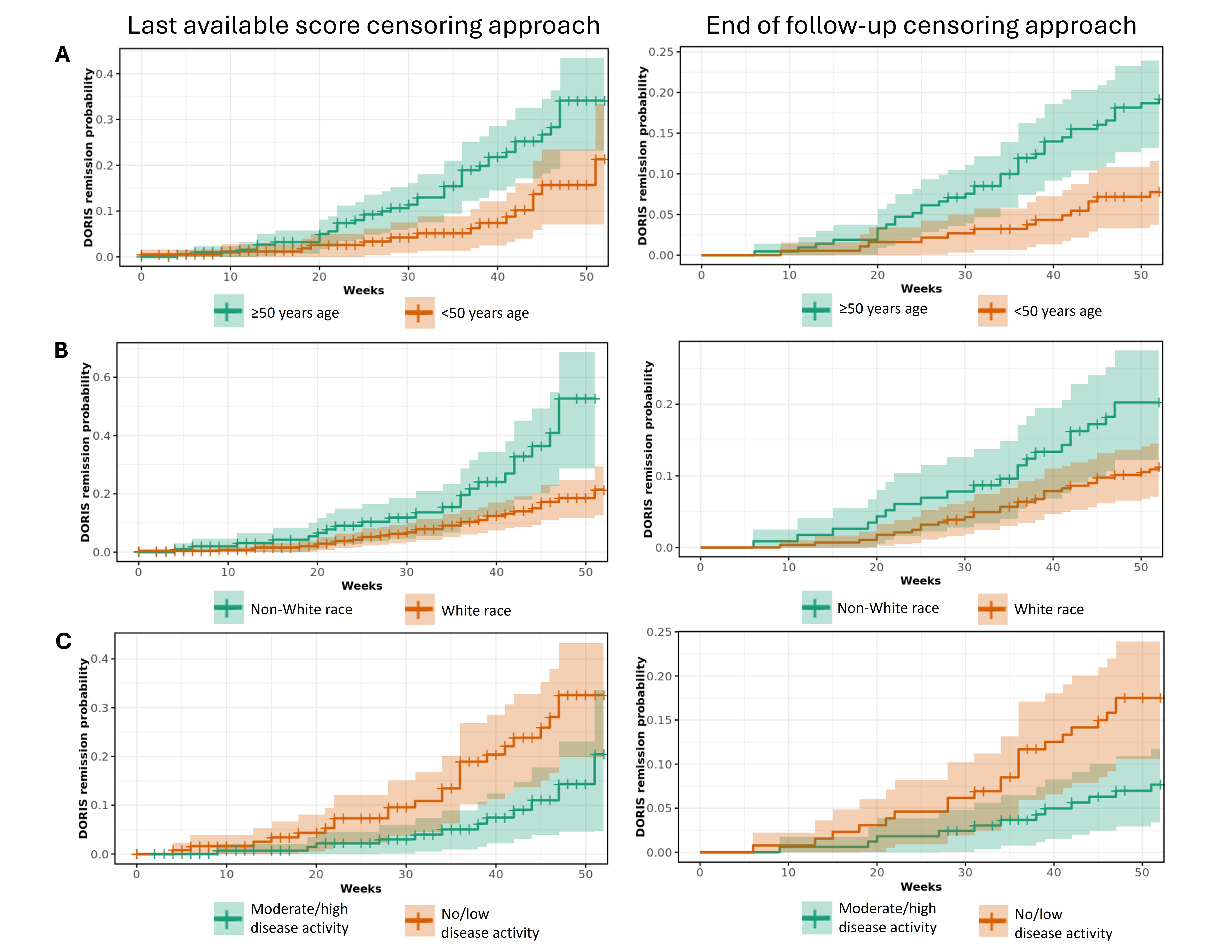 Figure 1. Unadjusted probability estimates of achieving real-world SLE remission (per DORIS proxy) stratified by (A) age, (B) race, and (C) baseline SLEDAI disease activity, as assessed by two censoring approaches.
Figure 1. Unadjusted probability estimates of achieving real-world SLE remission (per DORIS proxy) stratified by (A) age, (B) race, and (C) baseline SLEDAI disease activity, as assessed by two censoring approaches.
Shaded areas represent confidence bounds.
.jpg) Figure 2. Logistic regression model with real-world SLE remission (per DORIS proxy) by 52 weeks as the event, and baseline patient demographics and clinical characteristics as covariates.
Figure 2. Logistic regression model with real-world SLE remission (per DORIS proxy) by 52 weeks as the event, and baseline patient demographics and clinical characteristics as covariates.
Note: No/low disease activity per SLEDAI is not necessarily a complete measure of disease status, as all patients were determined by their physician to need initiation of a new therapy (biologic).
CCI, Charlson Comorbidity Index.
To cite this abstract in AMA style:
Patel A, Gennarelli R, Bello T, Bonakdar A, Worley K. Predictors of Real-World Remission in Patients with SLE Initiating Belimumab in the USA [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/predictors-of-real-world-remission-in-patients-with-sle-initiating-belimumab-in-the-usa/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-real-world-remission-in-patients-with-sle-initiating-belimumab-in-the-usa/
