Session Information
Date: Tuesday, October 28, 2025
Title: (2377–2436) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Deucravacitinib is an oral, selective, allosteric TYK2 inhibitor currently under investigation for the treatment of active discoid and/or subacute cutaneous lupus erythematosus (DLE and SCLE) and systemic lupus erythematosus (SLE). A need exists for patient-centered evidence on meaningful improvement in trials, as current measures may not fully capture patient priorities or experiences (Guo LN, et al. Lupus Sci Med 2021;8:e000529). This embedded qualitative interview study aimed to understand DLE/SCLE symptoms, signs, and meaningful changes from the patient perspective, and to link these insights with clinical endpoints to better inform the interpretation of meaningful change.
Methods: PAISLEY DLE/SCLE (NCT04857034) was a global, multicenter, phase 2, randomized, double-blind, placebo-controlled trial that evaluated the safety and efficacy of deucravacitinib in patients aged 18–75 years with biopsy-confirmed DLE/SCLE per 2019 EULAR/ACR classification with or without SLE. Patients were randomized 1:1:1 to 1 of 2 deucravacitinib doses (3 or 6 mg BID) or placebo and evaluated every 4 weeks through Week 16.The primary endpoint was percentage change from baseline in the Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) score at Week 16. Qualitative interviews were planned to be conducted within 21 days after the Week-16 visit using a semi-structured interview guide to assess the impact on symptoms/signs and identified meaningful change. Interviews were thematically analyzed and allowed for the linkage of patient-reported experiences with clinical outcomes. Descriptive statistics were calculated for quantifiable variables.
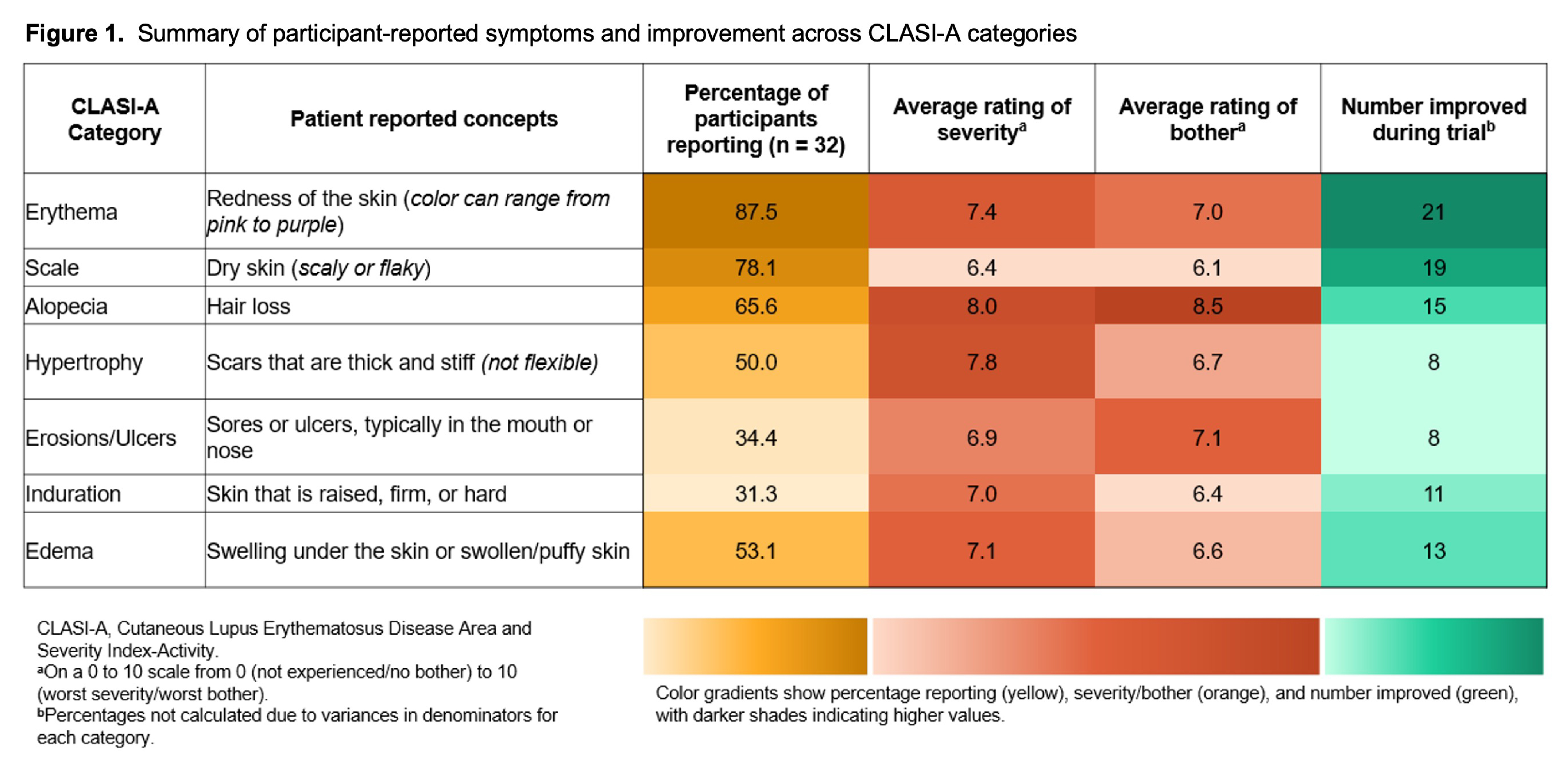
Results: A total of 32 patients from all treatment arms of the clinical trial were interviewed, with a mean (SD) age of 44.2 (13.6) years (Table 1). Most participants were classified as having moderate DLE/SCLE at baseline, with a mean (SD) CLASI-A score of 17.7 (7.5), which improved to mild DLE/SCLE at Week 16 (3.9 [4.7]). Perceived symptoms/signs affecting patients before or during the trial included erythema (87.5%), scale (78.1%), and alopecia (65.6%) (Figure 1). When participants were asked to rate overall severity and bothersomeness on a 0–10 scale from least to most extreme, the highest mean rating for both severity and bothersomeness was seen in alopecia (8.0 and 8.5, respectively). Participants were asked to describe their overall symptom/sign severity at its worst and the desired meaningful change after treatment on an 11-point scale from 0 (none) to 10 (extremely severe). For participants with complete data (n = 19), a mean desired change of 54% was considered meaningful. Actual changes in CLASI-A scores ranged from 30% to 100% improvements, with a mean 78% improvement (Table 2). Meaningful improvement defined as achieving ≥50% CLASI-A score improvement was reported in 89.5% of participants, while 78.9% achieved meaningful improvements based on their desired change in overall symptom/sign severity.
Conclusion: CLASI-A captures symptoms/signs relevant to patients with DLE/SCLE, and patient-defined meaningful change was consistent with clinically meaningful CLASI-A thresholds.
To cite this abstract in AMA style:
Werth V, Hobar C, Agrawal R, Kimel M, Martin M, Correll J, Choi J, Christodoulou A, Eliason L, Merola J. Relevant Symptoms and Signs of Active Discoid and/or Subacute Cutaneous Lupus Erythematosus Among Participants in a Phase 2 Clinical Trial: Patient-Embedded Qualitative Interviews [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/relevant-symptoms-and-signs-of-active-discoid-and-or-subacute-cutaneous-lupus-erythematosus-among-participants-in-a-phase-2-clinical-trial-patient-embedded-qualitative-interviews/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/relevant-symptoms-and-signs-of-active-discoid-and-or-subacute-cutaneous-lupus-erythematosus-among-participants-in-a-phase-2-clinical-trial-patient-embedded-qualitative-interviews/

.jpg)
.jpg)