Session Information
Date: Tuesday, October 28, 2025
Title: (2377–2436) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Up to a third of those with suspected SLE progress to definite SLE; however, reliable predictive markers for disease progression remain unknown. Previously, we developed a Lupus Lymphocyte Activation Score (LLAS) based on key cell subsets (transitional B cells, age-associated B cells, plasmablasts, Tph cells, and Tfh cells) observed in those with or at risk of developing SLE (Horisberger, 2024). Here we examined the relationship between autoantibody profiles, including anti-dense fine speckled 70 (DFS70) antibodies, as well as sialic acid binding Ig like lectin 1 (SIGLEC1, a surrogate marker for type I interferon), LLAS, and SLE development among those with suspected disease.
Methods: Blood samples were collected from 45 patients with new onset ( < 3 yrs) suspected SLE at Brigham and Women’s Hospital Lupus Center. At baseline, subjects did not meet SLE classification criteria and received prednisone < 10mg/day and no immunosuppressants. Using baseline samples, SIGLEC1 was measured by flow cytometry on a subset of patients (n=20) and a comprehensive autoantibody profile on all patients: ANA by indirect immunofluorescence assay on HEp-2 cells and patterns classified according to the International Consensus on ANA Pattern anti-cell (AC) nomenclature, SLE-related autoantibodies, including anti-DFS70 by a fully automated multi-analyte system using particle-based multi-analyte technology, and anti-C1q and anti-phospholipid antibodies by ELISA. To examine patterns of autoantibodies, we used hierarchical clustering and investigated their relationships with disease progression, LLAS, and SIGLEC1 levels.
Results: Of 45 patients, 36 had multiple visits with a mean follow-up of 13.6 months. In follow-up, 3 patients were diagnosed with SLE and met 2012 SLICC or 2019 EULAR/ACR criteria; 4 were diagnosed with or suspected of new dermatomyositis or Sjögren’s syndrome. We identified 5 clusters of suspected SLE patients based on autoantibody profiles (Fig. 1A, B). Cluster A (AC-4 nuclear speckled with multiple autoantibody reactivity including anti-RNP, -dsDNA, -anti-Ro60/SSA) was more likely progress or develop a CTD including SLE (OR 1.94, 95%CI 0.42-3.46) than others (Fig. 2A), particularly compared to Cluster C (no autoantibodies) (diff. 43.43%, 95%CI 0.08%-0.79%). Cluster A also had significantly higher median SIGLEC1 expression on total CD14+ monocytes vs. Cluster D (monospecific antiDFS70 with AC2, diff. 388.00, 95%CI 86.78-689.22) and Cluster E (AC30, diff. 382.97, 95%CI 116.30-649.64). There was no difference in LLAS by autoantibody cluster (Fig. 2B).
Conclusion: Autoantibody profile characterized by nuclear speckled with multiple autoantibody reactivity, including anti-RNP, -dsDNA, and -Ro60/SSA, was predictive of CTD development. Although based on a smaller sample size, this cluster also had higher baseline SIGLEC1 vs. those with monospecific DFS70 antibodies or no autoantibodies. Further analysis will assess whether LLAS, SIGLEC1, and autoantibodies are complementary, and when combined, could enhance the prediction of SLE development.
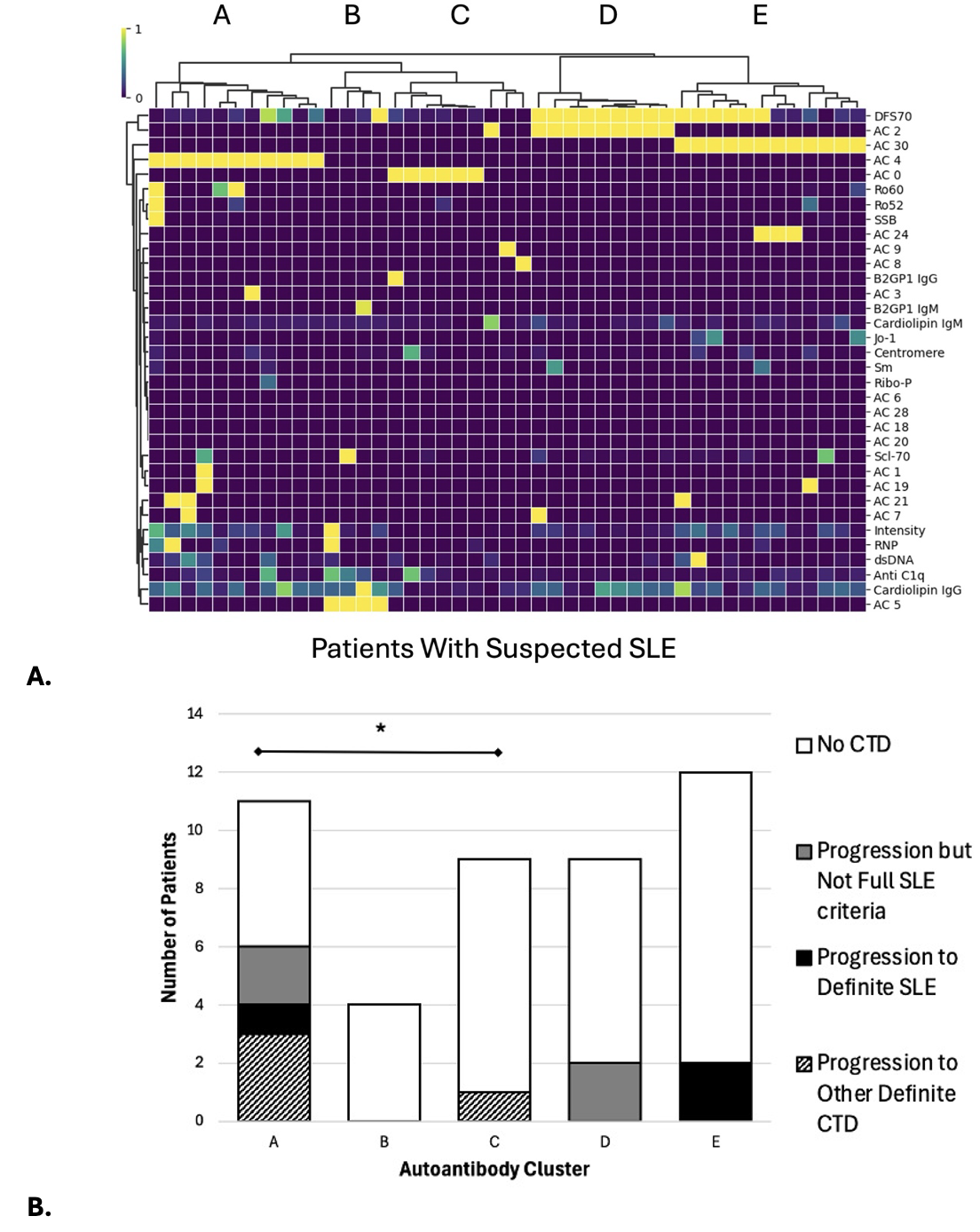 Figure 1. A) Five autoantibody clusters identified based on baseline samples of 45 samples of suspected SLE (left to right): Cluster A (n=11) AC-4 nuclear speckled pattern with the greatest autoantibody reactivity including anti-RNP, anti-dsDNA, and anti-Ro60/SSA, Cluster B (n=4) nuclear large speckled pattern, Cluster C (n=9) mostly no autoantibodies, Cluster D (n=9) monospecific anti-DFS70 antibody with dense fine speckled (AC2) pattern, and Cluster E (n=12) nuclear speckled with mitotic plate staining (AC-30). The ANA patterns were classified according to the most recently updated International Consensus on ANA Patterns recommendations and performed on Indirect immunofluorescence assay (IFA) using HEp-2 substrate (NovaLite,Werfen, San Diego, USA). In accord with the manufacturers’ directions, a positive test was defined as a titer of >=1:80. IFA results (titres and patterns) were initially read by an automated digital IFA microscope (NovaView, Werfen) and then validated by technologists with >15 years of experience. SLE-related antibodies and anti-DFS70 antibodies were performed on the Aptiva® Connective Tissue Disease Essential kit, a fully automated multi-analyte system using particle-based multi-analyte technology (Werfen, San Diego, USA). IgG and IgM anti-cardiolipin and IgG and IgM anti–β2-glycoprotein-1 (β2GP1) and anti-C1q antibodies were performed using an ELISA (Werfen, San Diego, USA). B) Cluster A (AC-4 nuclear speckled pattern and high autoantibody reactivity) had the largest number of patients with disease progression +/- definite SLE or other CTD at follow-up. *denotes a significant difference in proportion of patients who had disease progression +/- definite SLE or other CTD at follow-up (Cluster A vs. C, diff. 43.43%, 95%CI 0.08%-0.79%).
Figure 1. A) Five autoantibody clusters identified based on baseline samples of 45 samples of suspected SLE (left to right): Cluster A (n=11) AC-4 nuclear speckled pattern with the greatest autoantibody reactivity including anti-RNP, anti-dsDNA, and anti-Ro60/SSA, Cluster B (n=4) nuclear large speckled pattern, Cluster C (n=9) mostly no autoantibodies, Cluster D (n=9) monospecific anti-DFS70 antibody with dense fine speckled (AC2) pattern, and Cluster E (n=12) nuclear speckled with mitotic plate staining (AC-30). The ANA patterns were classified according to the most recently updated International Consensus on ANA Patterns recommendations and performed on Indirect immunofluorescence assay (IFA) using HEp-2 substrate (NovaLite,Werfen, San Diego, USA). In accord with the manufacturers’ directions, a positive test was defined as a titer of >=1:80. IFA results (titres and patterns) were initially read by an automated digital IFA microscope (NovaView, Werfen) and then validated by technologists with >15 years of experience. SLE-related antibodies and anti-DFS70 antibodies were performed on the Aptiva® Connective Tissue Disease Essential kit, a fully automated multi-analyte system using particle-based multi-analyte technology (Werfen, San Diego, USA). IgG and IgM anti-cardiolipin and IgG and IgM anti–β2-glycoprotein-1 (β2GP1) and anti-C1q antibodies were performed using an ELISA (Werfen, San Diego, USA). B) Cluster A (AC-4 nuclear speckled pattern and high autoantibody reactivity) had the largest number of patients with disease progression +/- definite SLE or other CTD at follow-up. *denotes a significant difference in proportion of patients who had disease progression +/- definite SLE or other CTD at follow-up (Cluster A vs. C, diff. 43.43%, 95%CI 0.08%-0.79%).
.jpg) Figure 2. Autoantibody clusters of suspected SLE patients and their A) median SIGLEC1 expression on total CD14+ monocytes (n=20 patients selected sequentially based on enrolment date). *Cluster A (n=5) had higher median SIGLEC1 expression compared to Cluster D (n=5) and Cluster E (n=6). Of note, there are no error bars for cluster C (n=1) as there was only one patient with SIGLEC1 level. Cluster B had 3 patients with SIGLEC1 measurements. B) Lupus Lymphocyte Activation Score (LLAS) (n=45) was not statistically different among different autoantibody clusters.
Figure 2. Autoantibody clusters of suspected SLE patients and their A) median SIGLEC1 expression on total CD14+ monocytes (n=20 patients selected sequentially based on enrolment date). *Cluster A (n=5) had higher median SIGLEC1 expression compared to Cluster D (n=5) and Cluster E (n=6). Of note, there are no error bars for cluster C (n=1) as there was only one patient with SIGLEC1 level. Cluster B had 3 patients with SIGLEC1 measurements. B) Lupus Lymphocyte Activation Score (LLAS) (n=45) was not statistically different among different autoantibody clusters.
To cite this abstract in AMA style:
Horisberger A, Oakes E, Dillon E, Adejoorin I, Caldropoli J, Marks K, Sasaki T, Moghaddam F, Sciore P, Fritzler M, Rao D, Choi M, Costenbader K. Autoantibody Clusters and SIGLEC1 are Predictive of Systemic Lupus Erythematosus Development [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/autoantibody-clusters-and-siglec1-are-predictive-of-systemic-lupus-erythematosus-development/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/autoantibody-clusters-and-siglec1-are-predictive-of-systemic-lupus-erythematosus-development/
