Session Information
Date: Tuesday, October 28, 2025
Title: (2377–2436) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The Type 1 & 2 SLE Model separates lupus disease activity into two groups: Type 1 (e.g. arthritis, rash, nephritis) and Type 2 SLE (e.g. widespread pain, fatigue, brain fog). A patient-reported outcome measure that accurately assesses Type 1 & 2 SLE activity would facilitate the monitoring of disease activity outside clinical settings. This study evaluates the utility of the LupusPRO in predicting Type 1 and Type 2 SLE activity.
Methods: Individuals meeting criteria for SLE were enrolled in a lupus registry. At routine visits, rheumatologists completed the SLEDAI and physician’s global assessments (PGA) for Type 1 and Type 2 SLE activity. Patients completed the Polysymptomatic Distress Score (PSD) and LupusPRO, which consists of 10 health-related quality of life (HRQoL) and 4 non-HRQoL domains scored from 0 (worst) to 100 (best).High Type 1 SLE activity was defined as SLEDAI ≥6, clinical SLEDAI ≥4, Type 1 PGA ≥1, or active nephritis. High Type 2 SLE activity was defined as PSD ≥8 or Type 2 PGA ≥1. Patients were categorized into 4 groups:Minimal SLE (low Type 1 and Type 2)Type 1 SLE (high Type 1 only)Type 2 SLE (high Type 2 only)Mixed SLE (high Type 1 and Type 2)Median LupusPRO domain scores were compared across groups by the Kruskal–Wallis test. Logistic regression models, using all LupusPRO domains as predictors, estimated predicted probabilities for high Type 1 and high Type 2 SLE separately. We compared models’ predicted probabilities with study definitions, calculated the proportion of correctly predicted outcomes, and calculated the Huberty’s I index ( >0.35 indicating predictive ability over chance)
Results: The LupusPRO was completed by 295 patients (mean age 44 years, 93% female, 59% Black). Among them, 36% had Minimal SLE activity, 11% Type 1 SLE activity, 29% Type 2 SLE activity, and 24% Mixed SLE activity. Differences in LupusPRO domain scores were observed across groups, with the Mixed SLE group having the lowest scores (Table 1). While scores were similar between the Type 2 SLE and Mixed SLE groups, lupus symptoms, lupus medications, emotional health and body image scores were significantly lower in the Mixed SLE group.LupusPRO domains accurately predicted Type 2 SLE activity in 82% of patients (Huberty’s I index = 0.64; Fig 1). Cognition, sleep and pain were key predictors of high Type 2 SLE activity. For Type 1 SLE activity, LupusPRO domains accurately predicted outcomes in 75% of patients (Huberty’s I index = 0.46), with higher accuracy for low Type 1 activity (91%) than high Type 1 activity (47%). Among patients with high Type 1 SLE activity classified as low Type 1 activity, nearly half had active nephritis. Lupus symptoms, procreation and emotional health were significant predictors of high Type 1 SLE activity.
Conclusion: The LupusPRO effectively captures unique aspects of HRQoL and non-HRQoL that patients with SLE experience, particularly patients with active Type 2 SLE. While it was less able to predict active Type 1 SLE, due to small sample size or active lupus nephritis presenting asymptomatically, the LupusPRO offers valuable insights into patient experiences. Utilizing the LupusPRO can enhance the care provided to SLE patients, addressing their specific concerns and better targeting treatment strategies.
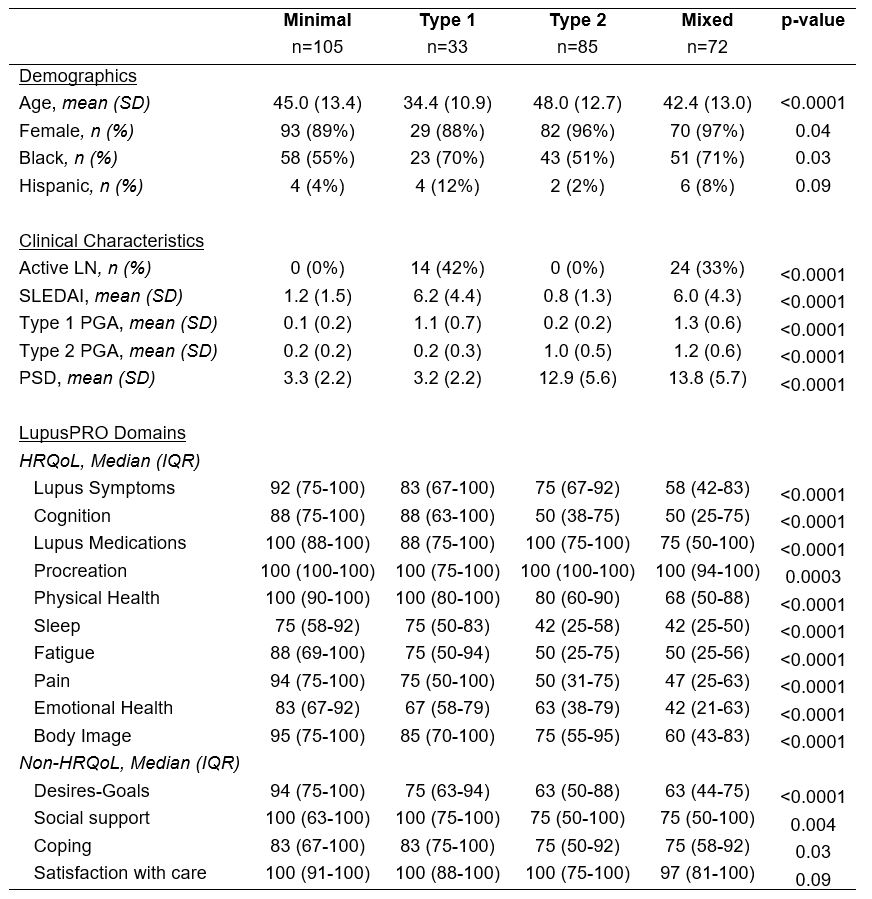 Table 1. Cohort characteristics and LupusPRO domains across Type 1 & 2 SLE activity groups.
Table 1. Cohort characteristics and LupusPRO domains across Type 1 & 2 SLE activity groups.
.jpg) Figure 1. Observed Versus Predicted Type 1 & 2 SLE Activity.
Figure 1. Observed Versus Predicted Type 1 & 2 SLE Activity.
To cite this abstract in AMA style:
Eudy A, Clowse M, Jolly M, Burshell D, Pisetsky D, Drake C, Somers T, Snyderman R, Sun K, Sadun R, Criscione-Schreiber L, Maheswaranathan M, Harris N, Doss J, Rogers J. Predicting Type 1 and Type 2 SLE Activity Using the LupusPRO [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/predicting-type-1-and-type-2-sle-activity-using-the-lupuspro/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predicting-type-1-and-type-2-sle-activity-using-the-lupuspro/
