Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Vunakizumab, also known as SHR-1314, is a novel a recombinant humanized monoclonal antibody against interleukin-17A. A randomised, double-blind, phase 2/3 trial (NCT04840485) demonstrated a significant benefit of vunakizumab over placebo in active ankylosing spondylitis (AS) at week 16, with sustained improvements through 32 weeks. However, it remains unknown whether the effect of vunakizumab on AS varies according to disease course (DC). Here, we conducted a post-hoc analysis of this randomized, double-blind, phase 2/3 trial to assess the efficacy and safety of vunakizumab in patients with active AS stratified by DC (< 5 versus ≥ 5 years).
Methods: In the phase 2/3 trial, adult patients with active AS who had either an inadequate response, intolerance, or contraindication to non-steroidal anti-inflammatory drugs were enrolled. All patients in this study fulfilled the 1984 modified New York criteria for AS and also met the American College of Rheumatology classification criteria. Data for this analysis were obtained from those eligible patients who were randomly assigned to receive either vunakizumab 120 mg or placebo at weeks 0, 2, 4, 8 and 12; from week 16, all patients transitioned to treatment with vunakizumab 120 mg until week 32. The primary endpoint was the proportion of patients who met the Assessment of Spondyloarthritis International Society 20 (ASAS20) response criteria at week 16. Key secondary endpoints included ASAS40, ASAS5/6 and safety.
Results: A total of 440 patients were included in this analysis, of which 174 had a DC of ≥ 5 years (vunakizumab 120 mg, n = 116; placebo, n = 58) and 266 had a DC of < 5 years (vunakizumab 120 mg, n = 178; placebo, n = 88). At week 16, the ASAS20 response rates were 62.07% for vunakizumab versus 43.10% for placebo (OR 2.15, 95% CI 1.13 to 4.09, P = 0.0196) in patients with a DC of ≥ 5 years; and in patients with a DC of < 5 years, the corresponding rates were 67.98% versus 42.05% (OR 2.98, 95% CI 1.75 to 5.07, P < 0.0001). Moreover, patients switching from placebo to vunakizumab at week 16 showed improved ASAS20 at week 32 (62.50% for DC of ≥ 5 years; 66.20% for DC of < 5 years), which was comparable to those who continued on vunakizumab (68.10% and 78.09%, respectively). Similar improvements were also observed for ASAS40 and ASAS5/6 (Table 1). In terms of safety, during the 16-week period, 110 (94.83%) of 116 patients with a DC of ≥ 5 years in the vunakizumab group and 54 (93.10%) of 58 such patients in the placebo group had at least one treatment-emergent adverse event (TEAE); while among patients with a DC of < 5 years, TEAEs occurred in 164 (92.13%) of 178 patients and 82 (93.18%) of 88 patients, respectively. The overwhelming majority of TEAEs were mild to moderate, with the most common being upper respiratory infection (DC of ≥ 5 years: 31 [26.72%] for vunakizumab versus 20 [34.48%] for placebo; DC of < 5 years: 42 [23.60%] versus 20 [22.73%]). No treatment-related deaths were reported.
Conclusion: Vunakizumab 120 mg showed improved efficacy with a manageable safety profile in patients with active AS, irrespective of DC.
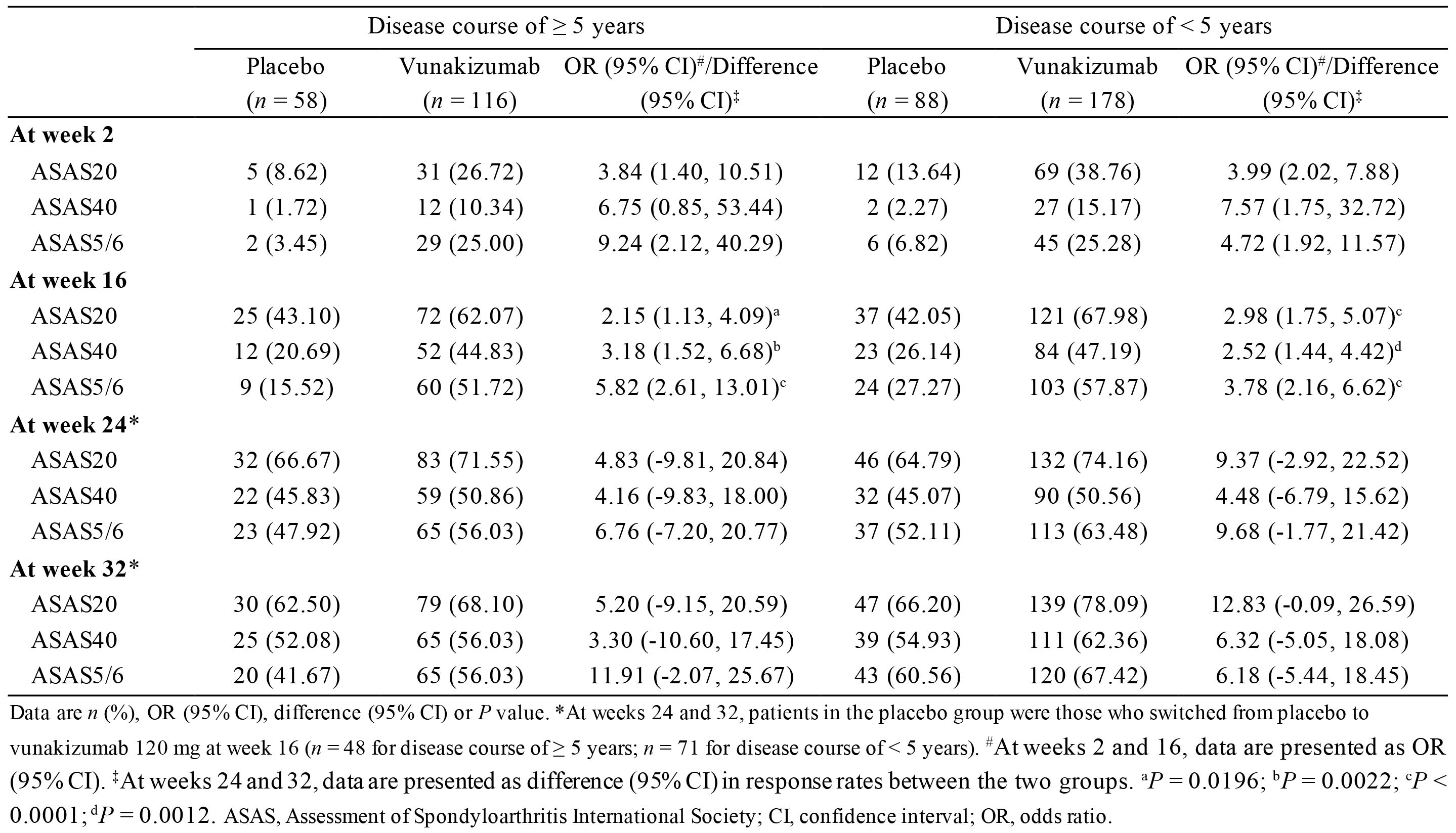 Table 1. ASAS response stratified by disease course
Table 1. ASAS response stratified by disease course
To cite this abstract in AMA style:
Shi X, Zhou L, Zhang H. Efficacy And Safety of Vunakizumab in Patients with Active Ankylosing Spondylitis Stratified by Disease Course: A Post-hoc Analysis of a Randomized, Double-Blind, Phase 2/3 Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-vunakizumab-in-patients-with-active-ankylosing-spondylitis-stratified-by-disease-course-a-post-hoc-analysis-of-a-randomized-double-blind-phase-2-3-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-vunakizumab-in-patients-with-active-ankylosing-spondylitis-stratified-by-disease-course-a-post-hoc-analysis-of-a-randomized-double-blind-phase-2-3-trial/
