Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: PsA affects male and female patients equally; however, variations in clinical manifestations and treatment responses between sexes have been reported. Deucravacitinib is an oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor under investigation for the treatment of PsA. This post hoc pooled analysis of 2 pivotal randomized, placebo (PBO)-controlled, phase 3 studies (POETYK PsA-1 [NCT04908202] and POETYK PsA-2 [NCT04908189]) evaluated the clinical efficacy of deucravacitinib vs PBO and patient-reported outcomes (PROs) by sex at W16 in patients with active PsA.
Methods: Patients in POETYK PsA-1 and PsA-2 were randomized to PBO or deucravacitinib 6 mg once daily (in PsA-2, a group included only as a safety reference arm received apremilast) through W16. The primary endpoint was ACR 20 at W16. Secondary endpoints included assessments of PsA disease activity, joints, skin, and quality of life vs PBO at W16. Binary data were analyzed using a logistic regression model that included treatment, sex, and treatment-sex interaction. Continuous data were analyzed using an ANCOVA model that included treatment, sex, baseline value, and treatment-sex interaction. P values were provided for the treatment-sex interaction. A P value > 0.05 indicated that the treatment effect between deucravacitinib and PBO was similar for male and female patients.
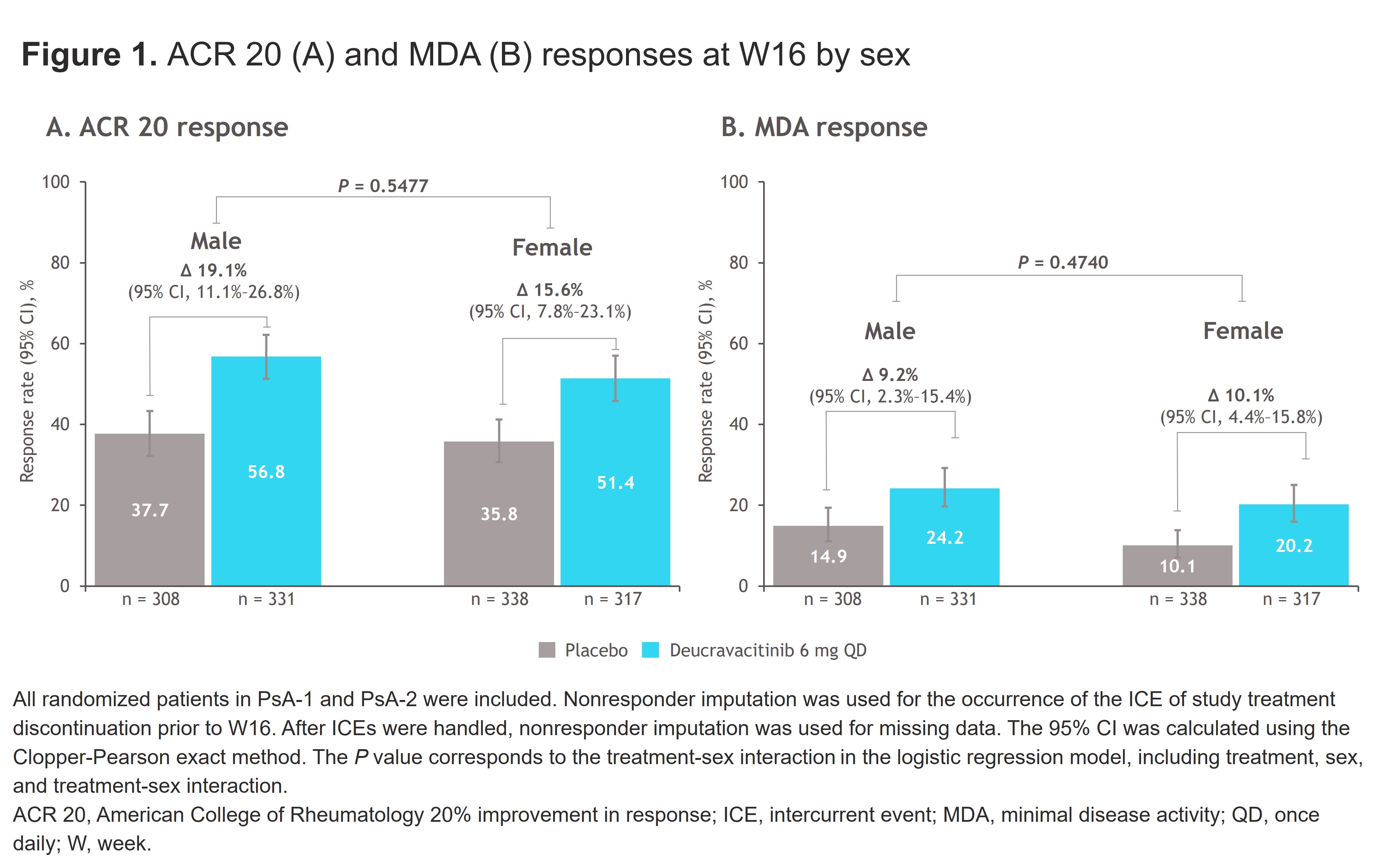
Results: Across POETYK PsA-1 and PsA-2, 646 patients were randomized to PBO (male, n = 308; female, n = 338), and 648 were randomized to deucravacitinib (male, n = 331; female, n = 317). At W16, ACR 20 was achieved in more patients treated with deucravacitinib vs PBO, with no difference between male and female patients (male, 56.8% vs 37.7%; female, 51.4% vs 35.8%; P = 0.5477) (Figure 1A). Similar trends in both male and female patients were observed for ACR 50 and ACR 70 responses. MDA was also achieved in more patients treated with deucravacitinib vs PBO at W16 (male, 24.2% vs 14.9%; female, 20.2% vs 10.1%; P = 0.4740) (Figure 1B). Response rates in patients treated with deucravacitinib were higher at W16 vs PBO for secondary endpoints of PASI 75, DAPSA and DAS28-CRP disease remission or low disease activity, enthesitis (SPARCC) resolution, and dactylitis resolution as well as a greater change from baseline (CfB) in DAPSA score in both male and female patients (Table 1). For PROs, more patients treated with deucravacitinib vs PBO achieved a clinically meaningful improvement in HAQ-DI score and showed greater CfB in HAQ-DI, SF-36 PCS and MCS, and FACIT-Fatigue scores at W16 in both male and female patients (Table 2).
Conclusion: Efficacy at W16 was similar for male and female patients who received deucravacitinib, and patients treated with deucravacitinib had superior efficacy vs PBO across multiple endpoints, including overall disease activity measures, musculoskeletal and dermatologic manifestations, and quality of life. Results of this post hoc pooled analysis were consistent with that of the overall POETYK PsA-2 population W16 results at a study level and those observed in the phase 2 study of deucravacitinib in active PsA in the overall population and by sex.
To cite this abstract in AMA style:
Eder L, Mease P, Merola J, Ogdie A, Deodhar A, Coates L, Varga S, Kalapala N, Jou Y, Vritzali E, Gossec L. Clinical Efficacy in Male and Female Patients With Active Psoriatic Arthritis Treated With Deucravacitinib: A Pooled Analysis of Pivotal Phase 3 Studies [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/clinical-efficacy-in-male-and-female-patients-with-active-psoriatic-arthritis-treated-with-deucravacitinib-a-pooled-analysis-of-pivotal-phase-3-studies/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-efficacy-in-male-and-female-patients-with-active-psoriatic-arthritis-treated-with-deucravacitinib-a-pooled-analysis-of-pivotal-phase-3-studies/

.jpg)
.jpg)