Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
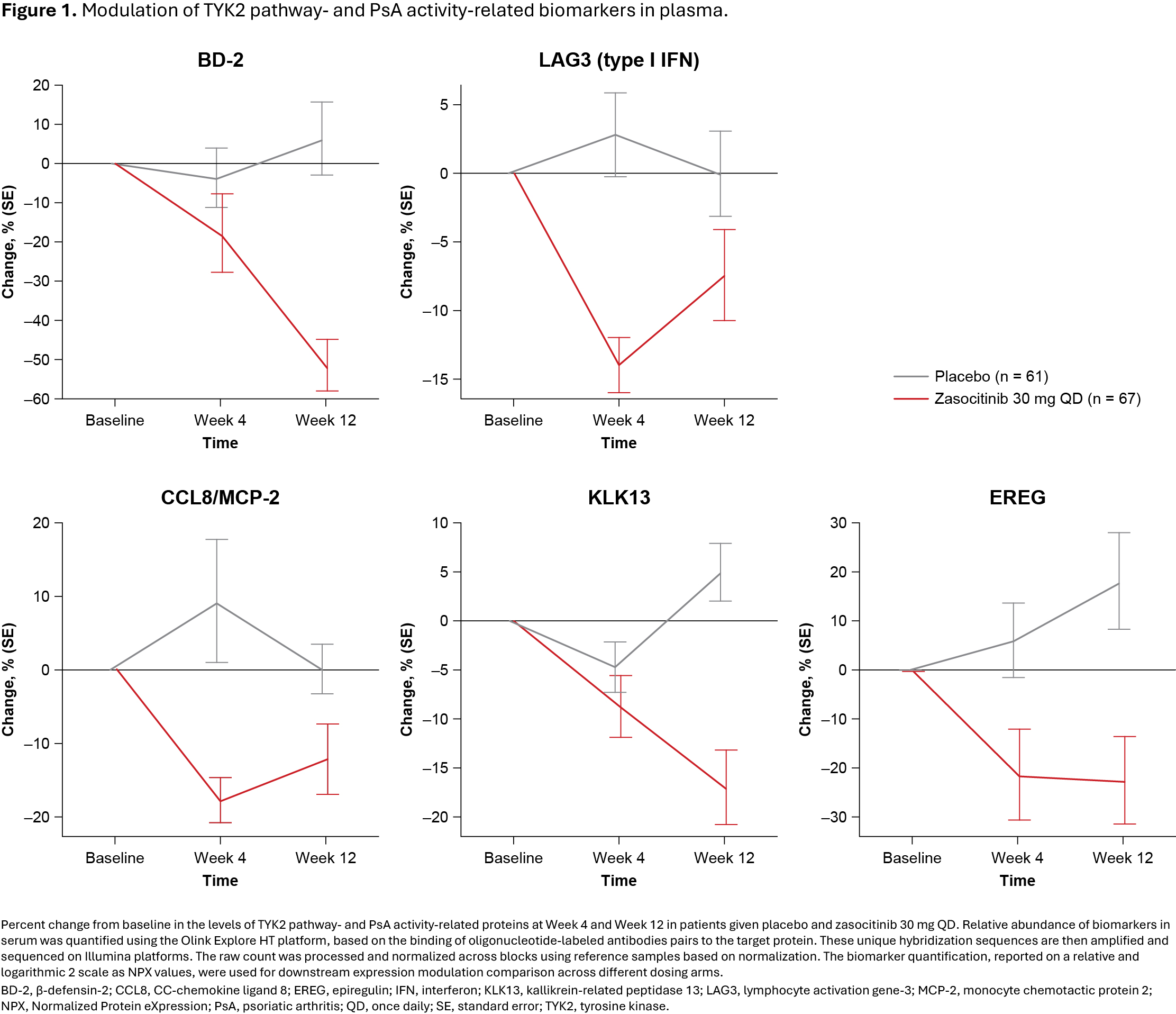
Background/Purpose: There is a significant unmet need for clinically informative biomarkers of disease activity and therapeutic response in immune-mediated inflammatory diseases (IMIDs). Tyrosine kinase 2 (TYK2) is a crucial mediator of pro-inflammatory pathways fundamental to the pathophysiology of some IMIDs, including psoriasis and psoriatic arthritis (PsA).Zasocitinib (TAK-279) is an investigational, oral, allosteric, highly selective, and potent TYK2 inhibitor for IMIDs. This post hoc analysis of a phase 2b trial of patients with active PsA evaluated the effect of zasocitinib 30 mg once daily (QD) on TYK2 pathway- and PsA activity-related biomarkers, and reported the association between baseline levels of biomarkers and American College of Rheumatology (ACR20) and Psoriasis Area and Severity Index (PASI 75) response.
Methods: In a phase 2b multicenter, double-blind, placebo-controlled, dose-ranging study, 290 patients with active PsA were randomized 1:1:1:1 to receive oral zasocitinib (5, 15, or 30 mg) or placebo QD for 12 weeks (NCT05153148). Blood samples were collected from patients in each group at baseline, Week 4, and Week 12 (pre-dose, if applicable). Concentrations of plasma biomarkers associated with the TYK2 pathway (β-defensin-2 [BD-2] and lymphocyte activation gene-3 [LAG3]) and with PsA (CC-chemokine ligand 8 [CCL8], kallikrein-related peptidase 13 [KLK13], epiregulin [EREG], interleukin [IL]-19, peptidase inhibitor 3 [PI3], IL-17C, IL-6, IL-22, and IL-17A) were measured using the Olink Explore HT. Biomarker levels in each treatment group were quantified at each timepoint; results for zasocitinib 30 mg are described.
Results: Zasocitinib 30 mg induced modulation of TYK2 pathway-related biomarkers (BD-2 and LAG3) and PsA activity related biomarkers (CCL8, KLK13, and EREG) as early as Week 4 and through Week 12 (p < 0.01; Figure 1); modulation of these biomarkers was numerically greater in ACR20 responders versus non-responders (p < 0.01; Figure 2a), and modulation of BD-2, IL-19, PI3, and IL-17C was numerically greater in PASI 75 responders versus non-responders (p < 0.01; Figure 2b).In patients with elevated baseline plasma levels of PI3, IL-6, BD-2, and IL-22, zasocitinib 30 mg treatment resulted in greater ACR20 response rates versus those receiving placebo (p < 0.01; Figure 3a). In patients with elevated baseline serum levels of BD-2, PI3, IL-17A, and IL-19, zasocitinib 30 mg treatment demonstrated greater PASI 75 response rates versus those receiving placebo (p < 0.01; Figure 3b).
Conclusion: In this phase 2b trial, zasocitinib 30 mg QD treatment led to modulation of key TYK2 pathway- and PsA activity-related biomarkers that mediate pathophysiology in patients with active PsA, particularly in ACR20 clinical responders. Baseline levels of key biomarkers may predict joint and skin responses to zasocitinib.
To cite this abstract in AMA style:
Choudhury A, Cheng J, Hong F, Kumar S, Tang J, Thakker P, Saha B, Arunachalam V, Hong T, Muensterman E, Wennbo H, McInnes I. Modulation of Tyrosine Kinase 2- and Disease-Related Biomarkers by Zasocitinib (TAK-279), an Oral, Allosteric, Highly Selective and Potent Tyrosine Kinase 2 Inhibitor, Is Associated With Clinical Response in Patients with Active Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/modulation-of-tyrosine-kinase-2-and-disease-related-biomarkers-by-zasocitinib-tak-279-an-oral-allosteric-highly-selective-and-potent-tyrosine-kinase-2-inhibitor-is-associated-with-clinical-resp/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/modulation-of-tyrosine-kinase-2-and-disease-related-biomarkers-by-zasocitinib-tak-279-an-oral-allosteric-highly-selective-and-potent-tyrosine-kinase-2-inhibitor-is-associated-with-clinical-resp/

.jpg)
.jpg)