Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Ianalumab, a glycoengineered, fully human, IgG1 mAb directed against B cell-activating factor (BAFF)-receptor (BAFF-R), targets B cells and their functions via a dual mechanism of action: depletion of B cells through enhanced antibody-dependent cellular cytotoxicity, and blockade of BAFF:BAFF-R–mediated signals. The purpose of this study is to better understand the dual mechanism of action of ianalumab in the blood and labial salivary gland (SG) tissue of patients with Sjögren’s disease (SjD).
Methods: This is an open-label, single-center, non-randomized, phase 2 mechanistic study (NCT05124925) with a 5-week screening period, a 6-month treatment period, and a follow up period of up to 2 years after the last dose. Eligible participants included those who fulfilled the 2016 ACR/EULAR classification criteria for SjD with anti-Ro/SSA autoantibodies, EULAR Sjögren’s Syndrome Patient Reported Index score of ≥5, labial SG focus score (FS) of ≥0.3/4mm2, and B/B+T cell ratio of ≥0.2 at screening. This interim analysis reports the results of patients who completed the 24-week treatment period (ianalumab 300 mg monthly subcutaneous injection). The primary outcome was change in B/B+T cell ratio from baseline (BL) to week (W) 25. Other outcomes included mass cytometry and single-cell RNA sequencing of circulating immune cells, biomarkers of B cell activation, and labial SG histologic parameters, as well as clinical evaluations, safety, and tolerability.
Results: In the safety analysis set (Nf21), ianalumab was well-tolerated with no drug-related serious adverse events (AEs) and one AE leading to discontinuation. Overall, 17 participants completed 24-week open-label treatment, underwent paired screening and W25 labial SG biopsy, and were analyzed. Clinical outcomes and biomarkers of B cell hyperactivity improved numerically. Total CD19+ B cell counts in blood decreased by 99% vs BL. B cells were almost completely depleted in most subsets, notably in naïve B cells, but to a lower extent in double negative effector memory cells (89.5%) and plasmablasts (69.5%; Figure 1a). Median depletion was significantly correlated with median BAFF-R expression in these subsets at BL (p=0.008; Figure 1b). Gene expression analysis revealed downregulation of B cell signaling, proliferation, and chemotaxis, and upregulation of oxidative phosphorylation and mitochondrial/cellular stress in the remaining naive B cells present at W25. IFN pathway genes were downregulated in plasmablasts at W25. While the FS was slightly reduced, based on regression analyses, the B/B+T cell ratio was statistically significantly reduced by 41% (80% CI: 22, 55) and the B cell density was reduced by 84% (80% CI: 68, 91) at W25 vs BL (Figure 2).
Conclusion: Ianalumab 300 mg monthly treatment almost completely depleted B cells in the circulation, and to a large extent in labial SG tissue, in line with improvement in clinical and serological parameters at W25 vs BL. These observations suggest direct and relevant effects of ianalumab 300 mg monthly on the target tissue in patients with SjD. Additionally, ianalumab 300 mg monthly modified the phenotype of the remaining B cells in circulation, shedding new light on the dual mechanism of action of ianalumab.
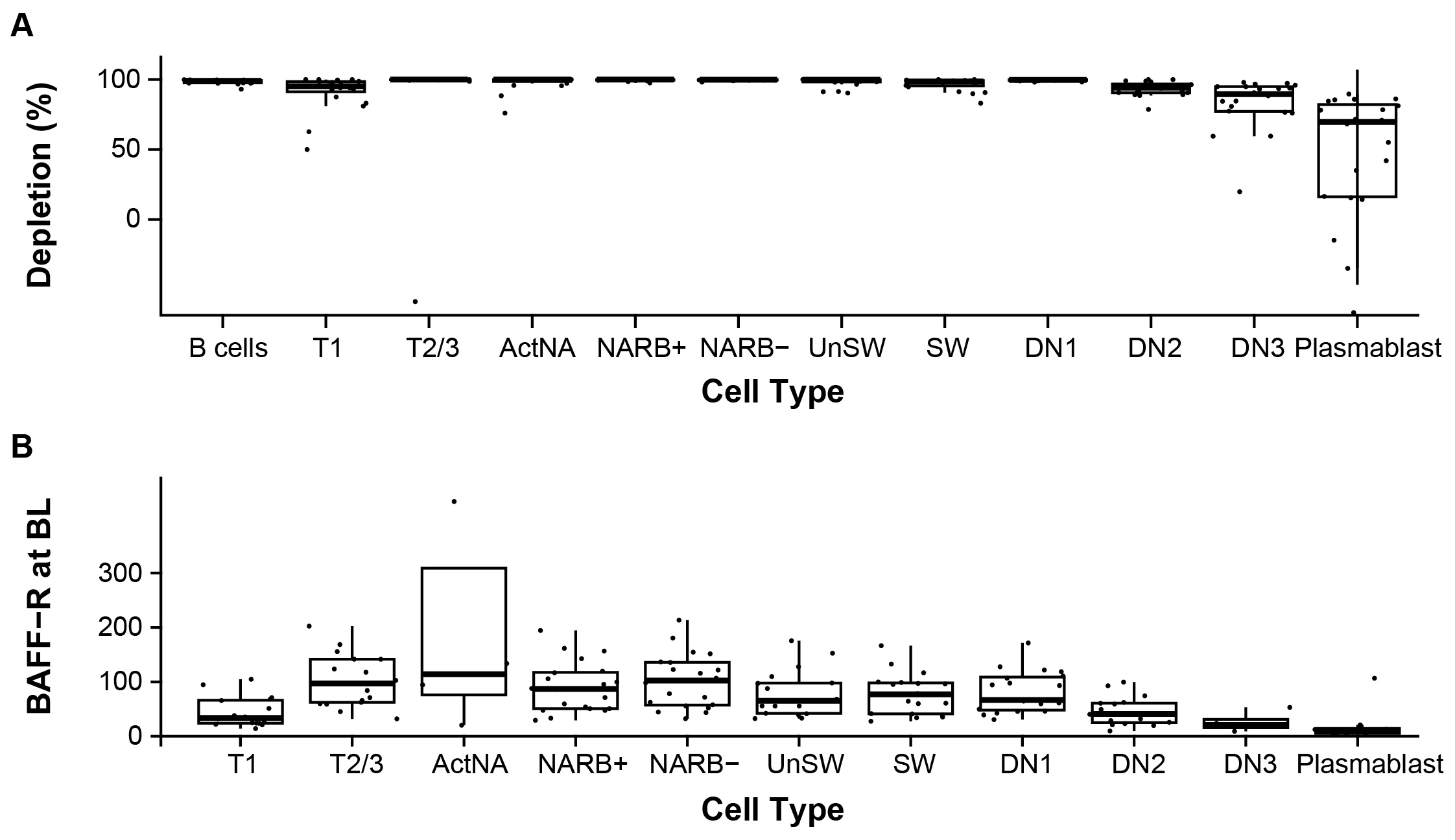 Figure 1. Extent of depletion of circulating B cells and B cell subsets at W25 (A) in relation to BAFF-R expression at BL (B).
Figure 1. Extent of depletion of circulating B cells and B cell subsets at W25 (A) in relation to BAFF-R expression at BL (B).
ActNA, activated naïve cells; BAFF-R, B cell-activating factor-receptor; BL, baseline; CD, cluster of differentiation; DN1–3, double negative effector memory cells; NARB; naïve cells (CD45RB); SW, switched memory cells; T1–3, transitional cells; UnSW, unswitched memory cells.
.jpg) Figure 2. Labial SG B/B+T cell ratio (A), B cell density in log scale of base 10 (B), and a representative example of immunohistochemistry staining for CD3/CD20 (C) at BL and W25.
Figure 2. Labial SG B/B+T cell ratio (A), B cell density in log scale of base 10 (B), and a representative example of immunohistochemistry staining for CD3/CD20 (C) at BL and W25.
BL, baseline; CD, cluster of differentiation; CV, coefficient of variation; SG, salivary gland; w, week.
To cite this abstract in AMA style:
Cornec D, Hillenbrand R, Hemon P, Saldana Miranda D, Le Rochais M, Santos Da Costa A, Frutoso M, Iperi C, Sommer U, Uguen A, Jamin C, Baley P, Denis C, Guellec D, Tison A, Strasser D, jousse s, Chen S, Giglioli N, SIPS C, SARAUX A, Siegel R, Hueber W, Hillion S, Devauchelle V, Bonal C. Evaluation of the Dual Mode of Action of Ianalumab (VAY736) in the Circulation and Salivary Gland Tissue of Patients With Sjögren’s Disease: Results From a Phase 2 Mechanistic Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-the-dual-mode-of-action-of-ianalumab-vay736-in-the-circulation-and-salivary-gland-tissue-of-patients-with-sjogrens-disease-results-from-a-phase-2-mechanistic-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-the-dual-mode-of-action-of-ianalumab-vay736-in-the-circulation-and-salivary-gland-tissue-of-patients-with-sjogrens-disease-results-from-a-phase-2-mechanistic-study/
