Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Sjögren’s syndrome is a heterogeneous autoimmune disease with no approved disease-modifying treatments. The aim of this systematic review was to evaluate primary Sjögren’s syndrome (pSS) patient characteristics included in clinical trials (CTs).
Methods: This systematic review was undertaken according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. The search was performed using PubMed, EMBASE, OVID, and ClinicalTrials.gov for phase II and III clinical trials of biologic drugs in pSS including ≥30 adult patients and published between 2010 and 2024. Baseline characteristics of participants and trial-related information were collected. Descriptive statistics were used for analysis.
Results: A total of 16 publications were eligible to be included in this review, with 87.5% being published after 2017. The CTs were predominantly randomized controlled trials with a placebo arm (93.8%) and phase II trials (62.5%). The mean follow-up period was 32 weeks. The majority of the CTs (56.3%) were fully sponsored by industry sources. A summary of the main characteristics of CTs selected is shown in Table 1.Inclusion criteria often focused on high disease activity, as measured by the ESSDAI score, with 62.5% requiring an ESSDAI score ≥5. Salivary flow measures were also common, used in 43.8% of the CTs. The primary outcomes were diverse, but 56.3% of the CTs focused on reducing ESSDAI scores.The CTs included in the analysis reported a total of 1607 patients, predominantly female (94%), with a mean age of 51.0 (4.04) years. The mean time from diagnosis was 6.47 (2.48) years. Racial diversity was underreported, with only 37.5% of CTs providing data on race. Among those, 77.8% of participants were White, 11.9% were Asian, and 5.2% were Black.Disease characteristics included high anti-SSA/Ro antibody positivity (92.9%), while other immunological and serological features are shown in Table 2. Mean ESSDAI score was 9.6 (2.8) and mean ESSPRI score was 6.3 (0.88). Other measurements of subjective symptoms notably a numerical rating scale (NRS) were also assed, including an NRS for dryness, fatigue and pain mean values of 7.2/10, 6.7/10 and 5.7/10, respectively. Patient-reported measures of disease burden are shown in Table 3.Concomitant treatments were common, with 87.5% of the CTs allowing simultaneous therapies. Despite most of the CTs being multicentric (75%), global representation was limited: only five CTs recruited patients in more than two continents and no CTs recruited in Africa.
Conclusion: This review highlights key challenges in pSS CTs: notably limited patient diversity, high subjective symptom burden, and heterogeneous outcomes measures. While the use of ESSDAI/ESSPRI is rising and reporting of race and ethnicity is improving, standardization remains incomplete. Earlier intervention and broader recruitment are needed to advance pSS therapies.
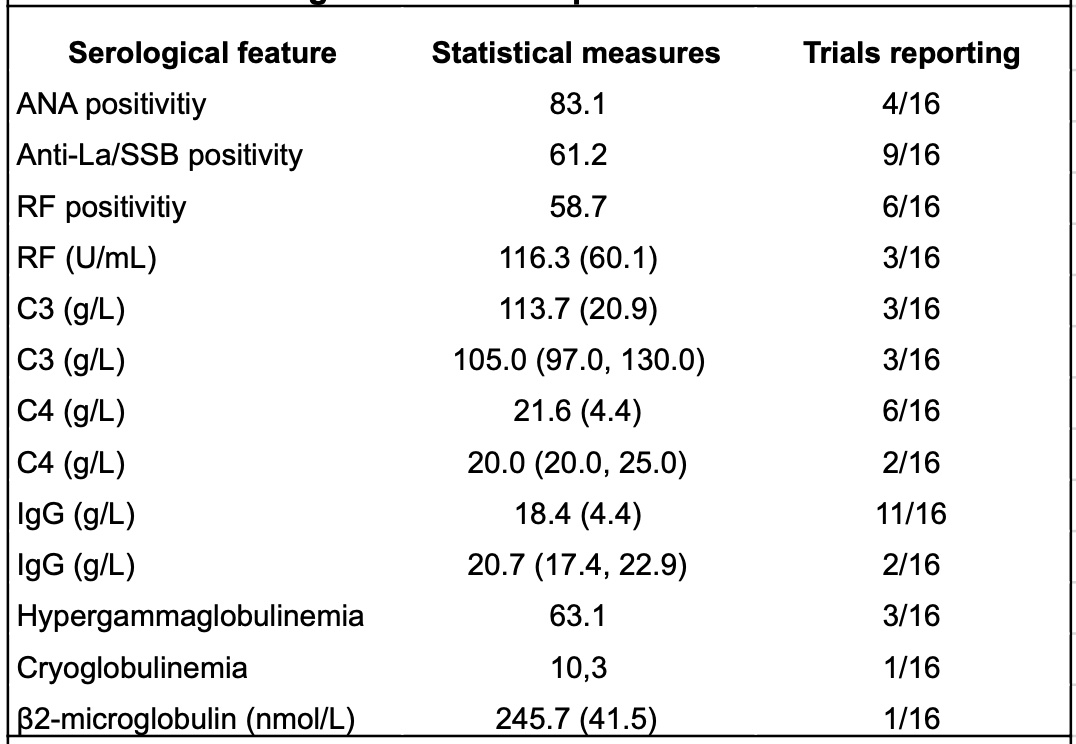 Table 1: Selected trials for biologics in pSS
Table 1: Selected trials for biologics in pSS
RCT: randomized clinical trial
.jpg) Table 2 : Serological features of patients included in the CTs
Table 2 : Serological features of patients included in the CTs
Data are mean (SD), median (min, max), or percentages.
ANA=antinuclear antibodies; anti-La/SSB= antibodies against Sjögren’s Syndrome B; RF=Rheumatoid Factor; IgG=Immunoglobulin G
.jpg) Table 3: Patient-reported measures of disease burden
Table 3: Patient-reported measures of disease burden
Data are mean (SD), median (min, max), or percentages.
SF-36: Short Form-36; PCS: Physical Component Summary of SF-36; MCS: Mental Component Summary of SF-36; MFI-20: Multidimensional Fatigue Inventory-20.
To cite this abstract in AMA style:
Navarro Joven C, Piunno S, Nikitsina M, SAN JOSE MENDEZ C, A. Isenberg D. Comparative Analysis of Patients Included in Trials Utilizing Biologic Drugs in the Treatment of Primary Sjögren’s Syndrome [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparative-analysis-of-patients-included-in-trials-utilizing-biologic-drugs-in-the-treatment-of-primary-sjogrens-syndrome/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-analysis-of-patients-included-in-trials-utilizing-biologic-drugs-in-the-treatment-of-primary-sjogrens-syndrome/
