Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Upadacitinib (UPA) is an oral, selective Janus kinase (JAK)-inhibitor proven effective and well-tolerated in patients with rheumatoid arthritis (RA) in 6 randomized clinical trials. The objective of the CLOSE-UP study was to evaluate the real-world effectiveness and safety of UPA among Canadian patients with prior exposure to conventional synthetic (cs)DMARD, biologic (b)DMARD, and targeted-synthetic (ts)DMARD experienced patients in real-world settings.
Methods: CLOSE-UP was a prospective, multicenter, observational post-marketing study in adults with moderate-to-severe RA who were treated with UPA 15 mg once daily, with the decision to initiate UPA therapy made prior to study participation. Patients were followed for 24 months after UPA initiation with data collected at routine clinic visits. The primary endpoint was the proportion of patients achieving a Disease Activity Score 28 Joint Count – C-reactive protein (DAS28-CRP) < 2.6 at 6 months. Secondary endpoints included pain score using a visual analog scale, fatigue (FACIT-F), physical function as measured by the Health Assessment Questionnaire-Disability Index (HAQ-DI) and other assessments of disease activity including Clinical Disease Activity Index (CDAI) score. Per protocol, eligible subjects were grouped by prior/most recent exposure to no b/tsDMARDs (bio-naïve); ≤2 bDMARDs but no tsDMARD (bio-experienced), and a maximum of 1bDMARD followed by a tsDMARD (tsDMARD-inadequate response [IR]). Data are presented as observed and summarized descriptively.
Results: Analysis included 412 patients, with 47.1% identified as bio-naïve, 41.3% as bio-experienced, and 10.7% as tsDMARD-IR (Table 1). At baseline, most patients (84.5%) exhibited a DAS28-CRP > 3.2. After 6 months of UPA treatment initiation, 60.7% of patients achieved a DAS28-CRP < 2.6 (primary endpoint) and 75.6% achieved a DAS28-CRP ≤ 3.2, (Figure 1a) with a response rate maintained for up to 24 months. Similar trends were observed using CDAI definitions for clinical remission and LDA. The type of prior/most recent DMARD exposure did not impact response rates. Additionally, the proportion of patients achieving a DAS28-CRP < 2.6 at the 6-month visit were similar between those on UPA monotherapy (62.7%) and those on UPA in combination with a csDMARD (60.1%). Improvements in pain score, fatigue, and physical function were observed throughout the study (Figure 1b). At 6 months, 86.5% of patients persisted in their treatment on UPA and 67.5% persisted up to Month 24. The safety profile of UPA was consistent with that seen in Phase 3 trials with no new safety signals (Table 2).
Conclusion: Consistent with clinical trial findings, this real-world Canadian study demonstrated a reduction in disease activity and improvements in patient-reported outcomes, supporting a favorable benefit-risk profile for patients treated with UPA. Notably, response rates were consistent irrespective of prior bDMARDs/tsDMARDs use or concomitant csDMARD.
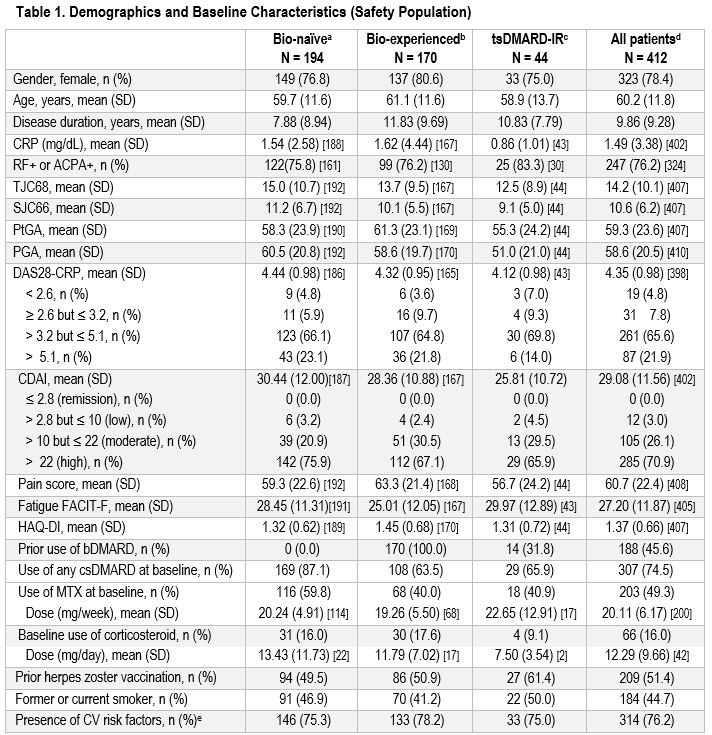 Abbreviations: ACPA+, anti-citrullinated protein antibodies positive; b, biologic; CDAI, Clinical Disease Activity Index; CRP, C-reactive protein; CV, cardiovascular; DAS28, Disease Activity Score – 28 Joint Count; DMARD, disease-modifying antirheumatic drugs; FACIT-F, Functional Assessment of Chronic Illness Therapy – Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; IR, inadequate response; MTX, methotrexate; N, sample size; n, number of patients in a category; PtGA, Patient’s Global Assessment; PGA, Physician’s Global Assessment; RF+, rheumatoid factor positive; SJC66, swollen 66 joint count; TJC68, tender 68 joint count; ts, targeted synthetic.
Abbreviations: ACPA+, anti-citrullinated protein antibodies positive; b, biologic; CDAI, Clinical Disease Activity Index; CRP, C-reactive protein; CV, cardiovascular; DAS28, Disease Activity Score – 28 Joint Count; DMARD, disease-modifying antirheumatic drugs; FACIT-F, Functional Assessment of Chronic Illness Therapy – Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; IR, inadequate response; MTX, methotrexate; N, sample size; n, number of patients in a category; PtGA, Patient’s Global Assessment; PGA, Physician’s Global Assessment; RF+, rheumatoid factor positive; SJC66, swollen 66 joint count; TJC68, tender 68 joint count; ts, targeted synthetic.
N are presented in square brackets [N] when different than in the header.
a – Have not been previously exposed to any bDMARD or tsDMARD.
b – Have not been previously exposed to tsDMARD and have been previously exposed to ≤ 2 bDMARDs.
c – Have been previously treated with 1 tsDMARD and ≤ 1 bDMARD prior to treatment with that tsDMARD.
d – Including 4 patients enrolled although it was discovered later they did not meet the eligibility criteria for the prespecified groups.
e – CV risk factors included past or current smoking, hyperlipidemia; body mass index ≥ 30, hypertension, diabetes, atherosclerosis, ischemic heart disease, and others.
.jpg) Abbreviations: CDAI, Clinical Disease Activity Index; CRP, C-reactive protein; cs, conventional synthetic; DAS28, Disease Activity Score – 28 Joint Count; DMARD, disease-modifying antirheumatic drugs; FACIT-F, Functional Assessment of Chronic Illness Therapy – Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; IR, inadequate response; MCID, minimal clinically important difference; N, sample size; ts, targeted synthetic; UPA, upadacitinib.
Abbreviations: CDAI, Clinical Disease Activity Index; CRP, C-reactive protein; cs, conventional synthetic; DAS28, Disease Activity Score – 28 Joint Count; DMARD, disease-modifying antirheumatic drugs; FACIT-F, Functional Assessment of Chronic Illness Therapy – Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; IR, inadequate response; MCID, minimal clinically important difference; N, sample size; ts, targeted synthetic; UPA, upadacitinib.
Bio-naïve patients have not been previously exposed to any bDMARD or tsDMARD.
Bio-experienced patients have not been previously exposed to tsDMARD and have been previously exposed to ≤ 2 bDMARDs.
tsDMARD-IR patients have been previously treated with 1 tsDMARD and ≤ 1 bDMARD prior to treatment with that tsDMARD.
a – MCID for FACIT-F was defined as a change from baseline ≥ 3.56; MCID for HAQ-DI was defined as a change from baseline ≥ 0.22.
.jpg) Abbreviations: MACE, major adverse cardiovascular events; NMSC, non-melanoma skin cancer; PY, patient-years; SAE, serious adverse events; TEAE, treatment-emergent adverse event; UPA, upadacitinib; VTE, venous thromboembolic events.
Abbreviations: MACE, major adverse cardiovascular events; NMSC, non-melanoma skin cancer; PY, patient-years; SAE, serious adverse events; TEAE, treatment-emergent adverse event; UPA, upadacitinib; VTE, venous thromboembolic events.
a – 1 patient had cardiac arrest (not related); 1 patient had acute myocardial infarction, COVID-19, acute kidney injury, and respiratory failure (all not related, main cause of death was identified as complications related to dialysis); 2 patients had pneumonia (1 possibly related, 1 not related)
To cite this abstract in AMA style:
Bessette L, Chow A, Rai R, Allard-Chamard H, Boulos P, Roy G, Liazoghli D. Effectiveness of Upadacitinib in Patients with Rheumatoid Arthritis in Canadian Real-World Practice: Final Results from the CLOSE-UP Post-Marketing Observational Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-upadacitinib-in-patients-with-rheumatoid-arthritis-in-canadian-real-world-practice-final-results-from-the-close-up-post-marketing-observational-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-upadacitinib-in-patients-with-rheumatoid-arthritis-in-canadian-real-world-practice-final-results-from-the-close-up-post-marketing-observational-study/
