Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Early use of targeted therapy (TT), such as anti-tumor necrosis factor (anti-TNF) biologics, show improved outcomes for RA1, but costs often limit access. Biosimilars, such as Sandoz adalimumab (SZ-ADL), are generally less expensive than their reference medicines and offer comparable efficacy2. Early initiation of anti-TNF biosimilar therapy could yield higher cost efficiency than conventional synthetic DMARDs (csDMARDs).yrThis study compared healthcare resource utilization (HCRU) and costs of RA patients (pts) receiving SZ-ADL as first line TT (1L) with csDMARD pts never prescribed a TT (TT-naïve).
Methods: Data were drawn from the Adelphi RA Disease Specific Programme (DSP™), a cross-sectional survey of rheumatologists and their next consulting pts with RA in a real-world clinical setting in France, Italy, Germany, Spain and the United Kingdom between July 2023 and August 2024. Rheumatologists, referring to pt medical records, provided information on demographics, disease and treatment history, HCRU and clinical outcomes.HCRU was assessed in SZ-ADL pts (1L) using inverse probability weighted regression adjustment (IPWRA) with csDMARD (TT-naïve) pts as a comparison group. Both groups had been receiving their therapy for ≥3 months. Pts were weighted for age, DAS-28 at treatment initiation, sex, Charlson Comorbidity Index, autoimmune comorbidities, total duration of prior csDMARDs and time from diagnosis to treatment initiation. Weighted regression adjusted for these variables and for treatment duration. Costs were sourced per country from diagnostic-related groups, national tariffs and government reports. Pre-weighting was performed to account for population and cost differences between countries. Results are reported as weighted means along with p-values.
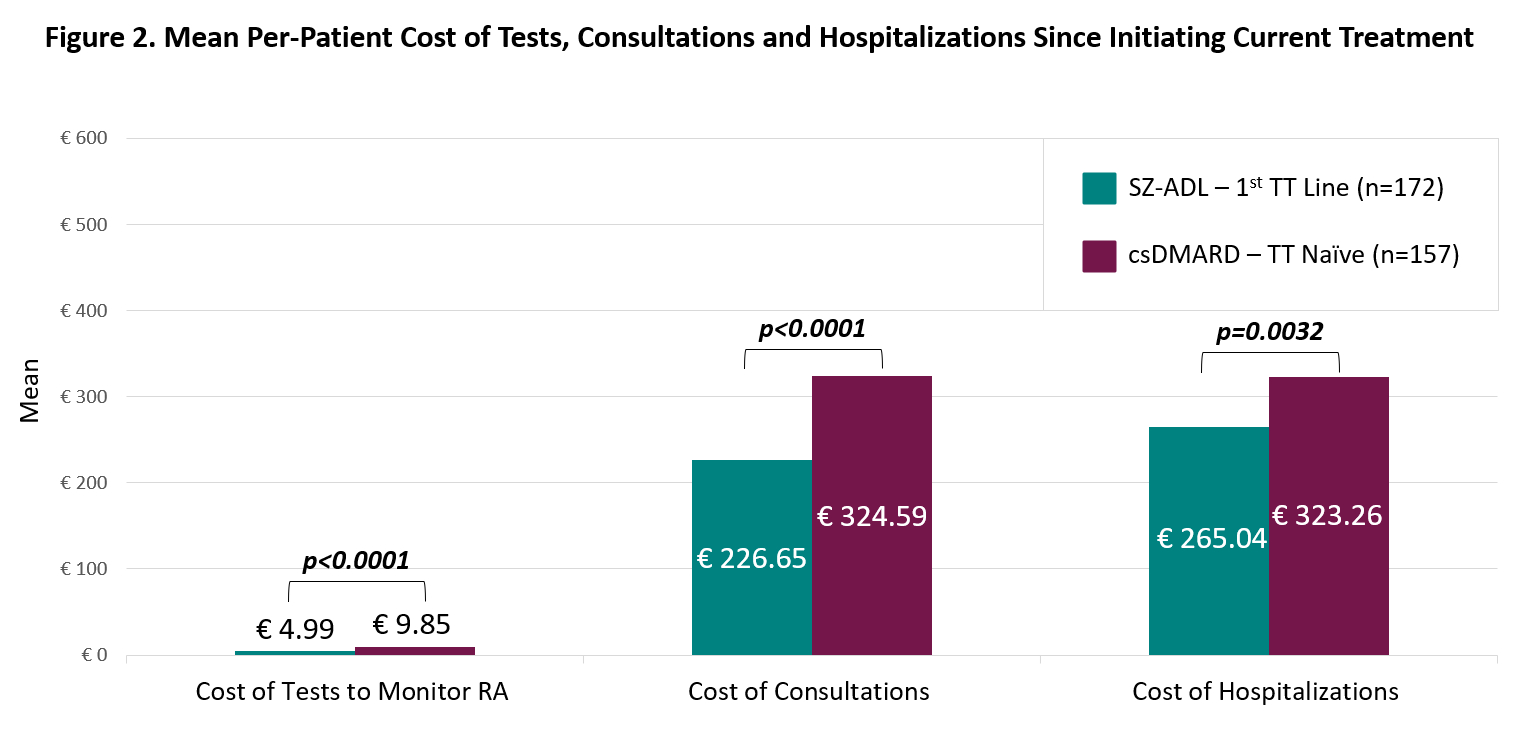
Results: A total of 329 pts were included in the analysis, 172 in the SZ-ADL and 157 in the csDMARD group. SZ-ADL pts had significantly fewer tests to monitor their RA during their treatment (8.59 vs 11.53, p < 0.0001) and fewer hospitalizations (0.19 vs 0.36, p=0.001) than csDMARD patients, but more consultations (4.00 vs 3.44, p < 0.0001) (Figure 1). This translated to significantly lower per-pt cost of tests to monitor RA (€4.99 vs €9.85, p < 0.0001), consultations (€226.65 vs €324.59, p < 0.0001) and hospitalizations (€265.04 vs €323.26, p=0.0032) for SZ-ADL pts (Figure 2).
Conclusion: Earlier initiation of SZ-ADL may provide cost savings to healthcare systems in Europe by reducing costs of testing, consultations and hospitalizations compared to pts remaining on csDMARDs. Residual confounding variables within the groups may be a potential limitation of the analysis.References:1Combe, B., Landewe, R., Daien, C.I., Hua, C., Aletaha, D., Álvaro-Gracia, J.M., Bakkers, M., Brodin, N., Burmester, G.R., Codreanu, C. and Conway, R., 2017. 2016 update of the EULAR recommendations for the management of early arthritis. Annals of the rheumatic diseases, 76(6), pp.948-959.2Baumgart, D.C., Misery, L., Naeyaert, S. and Taylor, P.C., 2019. Biological therapies in immune-mediated inflammatory diseases: can biosimilars reduce access inequities?. Frontiers in pharmacology, 10, p.279.
To cite this abstract in AMA style:
Aletaha D, Bremer D, Milligan J, Cao Z, Meadows R, Baraliakos X. Assessing the Real-World Impact of Earlier Initiation of Adalimumab vs. Conventional Synthetic DMARDs on Healthcare Resource Utilization in Patients with Rheumatoid Arthritis in Europe [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/assessing-the-real-world-impact-of-earlier-initiation-of-adalimumab-vs-conventional-synthetic-dmards-on-healthcare-resource-utilization-in-patients-with-rheumatoid-arthritis-in-europe/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessing-the-real-world-impact-of-earlier-initiation-of-adalimumab-vs-conventional-synthetic-dmards-on-healthcare-resource-utilization-in-patients-with-rheumatoid-arthritis-in-europe/

.jpg)