Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The outcome of Still’s disease (SD) has significantly improved due to new therapeutic options [37923864], early biologic initiation [24623686] and treat-to-target strategies [39317417]. Nonetheless, a substantial proportion of patients still experiences a refractory course, severely impacting their quality of life. Treatment strategies in these cases are not standardized and are affected by medication availability and clinician experience. This real-life study aims to describe current treatment practices and major unmet needs, fostering a uniform approach to refractory SD.
Methods: As part of the METAPHOR project, a PReS/PRINTO initiative to optimize therapy in SD and Macrophage Activation Syndrome (MAS), a global survey on refractory SD treatment was developed, with the following proposed subtypes: 1) persistent arthritis, 2) recurrent/refractory MAS and 3) SD-associated lung disease (SD-LD). Topics were selected by 22 expert pediatric rheumatologists, including 1 patient representative and 1 adult rheumatologist. The survey covered demographic data, clinical practice insights and a patient-focused section on unmet needs. International physicians part of the PReS/PRINTO network together with adult rheumatologists involved in SD care were invited to complete the web-survey between 3/12/24 and 14/2/25.
Results: A total of 206 physicians completed the survey, predominantly pediatric rheumatologists (91%), from 56 countries. Methotrexate was the most common 1st-line choice for SD-refractory arthritis, followed by anti-TNF agents and intra-articular steroids. JAK inhibitors (JAK-i) were considered mainly as 2nd- or 3rd-line option. If systemic symptoms co-occurred, 62% of respondents would modify their strategy, favoring methylprednisolone (MPN) pulses, JAK-i and ciclosporin. In SD patients with recurrent/refractory MAS, ciclosporin, anakinra, and MPN pulses were the most frequently selected medications. Treatment decisions were mainly guided by clinical severity, inflammatory markers, history of ICU admission and concomitant complications. Biomarkers played a minor role, partly due to limited access, reported by 22% of clinicians. For SD-LD, 41% would continue ongoing biologics, 30% withdraw and 29% decide case by case (figure 1). Factors influencing withdrawal included persistent disease activity, history of adverse reactions and impending MAS. HLA genotyping was rarely considered relevant (figure 2). The most used 1st-line agents for SD-LD included JAK-i, mycophenolate mofetil, and ciclosporin and only 8% would add Pneumocystis Jiroveci prophylaxis. Most respondents (82%) would consider Hematopoietic Stem-Cell Transplantation (HSCT) in difficult-to-treat SD patients, particularly for refractory MAS or SD-LD. Differences in management by clinical phenotype are detailed in figure 3.
Conclusion: Management for refractory SD is widely heterogeneous and varies by phenotype. JAK-i are the most commonly used agents alongside standard therapies and HSCT is increasingly considered a potential option, especially for refractory MAS and SD-LD. A consensus effort is essential to refine the definition and identify optimal treatment strategies for refractory cases.
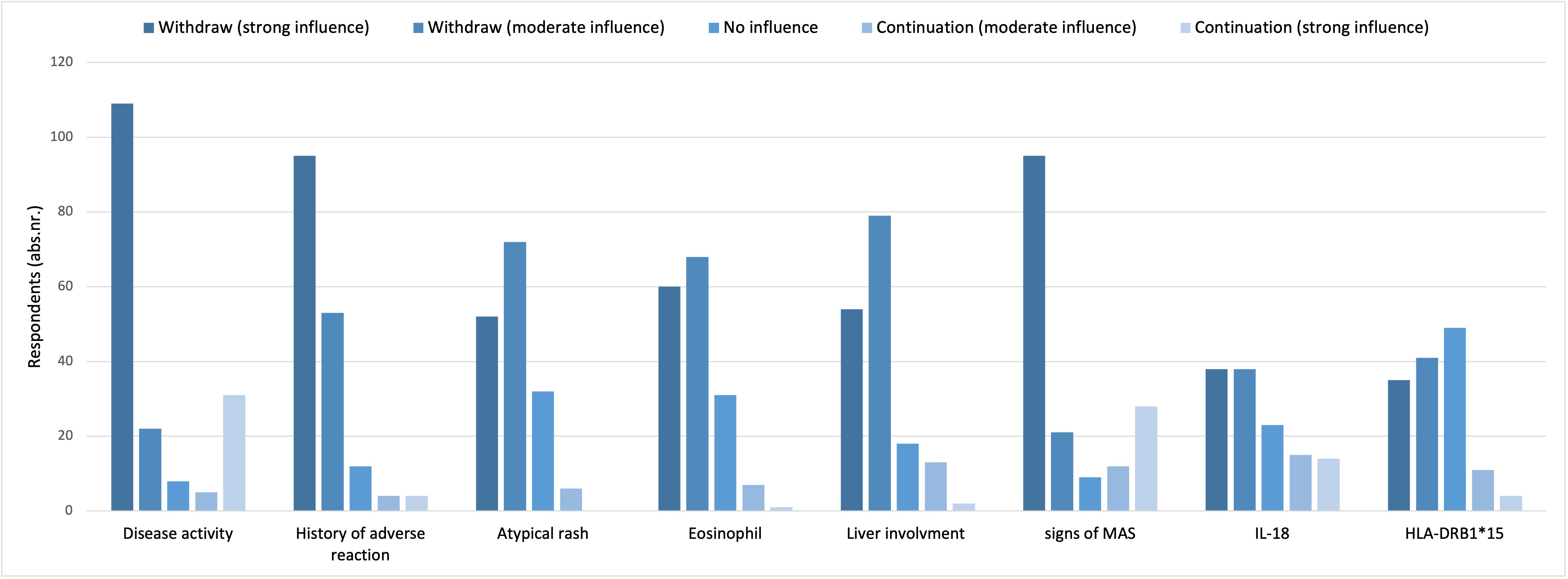 Figure 1 – Answer to the question “In a patient with Still disease and first detection of lung involvement while on therapy with IL-1 or IL-6 inhibitors, would you withdraw the ongoing biological treatment?”
Figure 1 – Answer to the question “In a patient with Still disease and first detection of lung involvement while on therapy with IL-1 or IL-6 inhibitors, would you withdraw the ongoing biological treatment?”
.jpg) Figure 2 – Answer to the question: “In a patient with Still disease and first detection of lung involvement while on therapy with IL-1 or IL-6 inhibitors, which parameters influence your decision regarding withdrawal/continuation of IL-1 or IL-6 blockade”
Figure 2 – Answer to the question: “In a patient with Still disease and first detection of lung involvement while on therapy with IL-1 or IL-6 inhibitors, which parameters influence your decision regarding withdrawal/continuation of IL-1 or IL-6 blockade”
.jpg) Figure 3 – Top 4 most commonly used first line agents for each subtype of refractory SD
Figure 3 – Top 4 most commonly used first line agents for each subtype of refractory SD
To cite this abstract in AMA style:
Rogani G, Baldo F, Bracaglia C, Foell D, Gattorno M, Jelusic M, Anton J, Brogan P, Canna S, Cron R, De Benedetti F, Grom A, Heshin Bekenstein M, Horne A, Khubchandani R, Mizuta M, Özen S, Quartier Dit Maire P, Ravelli A, Shimizu M, Schulert G, Scott C, Sinha R, Ruperto N, Swart J, Fautrel B, Vastert S, Minoia F. Real-Life Treatment Strategies for Refractory Still’s Disease: Results from a Worldwide Survey, the METAPHOR Project [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-life-treatment-strategies-for-refractory-stills-disease-results-from-a-worldwide-survey-the-metaphor-project/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-life-treatment-strategies-for-refractory-stills-disease-results-from-a-worldwide-survey-the-metaphor-project/
