Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Chronic nonbacterial osteomyelitis (CNO) is a noninfectious autoinflammatory bone disease which remains a diagnosis of exclusion, as existing diagnostic criteria are not widely accepted. Recently, the European Alliance of Associations for Rheumatology (EULAR) and the American College of Rheumatology (ACR) developed new classification criteria for CNO, demonstrating 82% sensitivity and 98% specificity in an initial study.We aimed to investigate sensitivity and specificity of the new EULAR/ACR classification criteria for pediatric CNO, in a distinct real-world clinical setting and compare it to the sensitivity and specificity of the existing Jansson and Bristol diagnostic criteria.
Methods: This was a single-center cross-sectional study including children ≤18 years, diagnosed with CNO, acute infectious osteomyelitis (AOM) and bone malignancy from June 1, 2018 to May 31, 2024. Patients with other mimicking conditions, immunodeficiency, sickle cell disease, or those previously included in the original development cohort were excluded. Patients were divided into two groups: a CNO group and a non-CNO group (AOM and bone malignancy), from the latter a representative, random, sample was selected (www.random.org). Demographic, clinical, laboratory, imaging and pathology data were collected at disease onset. EULAR/ACR criteria were retrospectively applied to the entire cohort, independently from the initial diagnosis. Patients with an aggregate score ≥ 55 points were classified as having CNO. A sensitivity analysis was also conducted where patients with criteria with missing data were excluded. Classification results using the new criteria were compared with the final clinical diagnosis based on physician assessment (criterion standard). The same analysis was applied to Jansson and Bristol criteria. From contingency tables, sensitivity and specificity were calculated.
Results: Of the 164 children included, 82 had CNO, 41 were randomly selected from 274 cases of AOM, and 41 from 849 cases of bone malignancy. The median age was 10 years (IQR 3–16), with 62% girls and 37% boys, 66% had a bone biopsy. Overall, 40% scored ≥ 55 and 60% did not, with 3 false positive and 19 false negative (Table 1). The EULAR/ACR criteria demonstrated 77% sensitivity and 96% specificity, with a positive predictive value (PPV) of 95% and negative predictive value (NPV) of 80%. In our sensitivity analysis excluding patients with incomplete data, results remained consistent (sensitivity 79%, specificity 96%, PPV 95%, NPV 81%). In comparison, the Jansson criteria showed 78% sensitivity, 67% specificity, PPV of 70%, and NPV of 75%. The Bristol criteria yielded 89% sensitivity, 70% specificity, PPV of 86%, and NPV of 74% (Table 2).
Conclusion: Based on its favorable sensitivity and specificity, especially in comparison to existing criteria, the new EULAR/ACR criteria appeared to be more effective in distinguishing CNO from AOM and bone malignancy at disease onset. These results were consistent with findings from the original validation cohort.
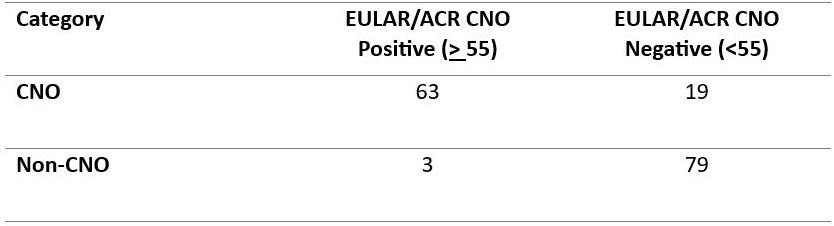 Table 1. Contingency Table of EULAR/ACR Criteria Performance in Classifying Pediatric CNO
Table 1. Contingency Table of EULAR/ACR Criteria Performance in Classifying Pediatric CNO
.jpg) Table 2. Comparison of Diagnostic Performance Across CNO Classification Criteria
Table 2. Comparison of Diagnostic Performance Across CNO Classification Criteria
To cite this abstract in AMA style:
Mastrangelo G, Itzkovitz E, Sawicka K, Goh I, Bitnun A, Hopyan S, Nathan P, laxer R, Feldman B. Confirming The Validity Of The New EULAR/ACR Classification Criteria For Pediatric Chronic Nonbacterial Osteomyelitis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/confirming-the-validity-of-the-new-eular-acr-classification-criteria-for-pediatric-chronic-nonbacterial-osteomyelitis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/confirming-the-validity-of-the-new-eular-acr-classification-criteria-for-pediatric-chronic-nonbacterial-osteomyelitis/
