Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The rarity of childhood-onset Takayasu arteritis (c-TAK) has resulted in small cohorts for study that has limited the potential for evaluating its course and outcomes. The objective of this study is to engage Pediatric Rheumatologists internationally to contribute patient data to a research network targeted to characterizing c-TAK and improving disease outcomes. This will be achieved through the following: Aim 1: Create an international c-TAK registry, Aim 2: Evaluate the performance of existing disease activity measures, and Aim 3: Create and validate a preliminary disease activity score specifically for c-TAK.
Methods: Member sites of Childhood Arthritis Rheumatology Research Alliance (CARRA) and Pediatric Rheumatology European Society (PReS) are invited to enroll patients in this c-TAK registry. The registry is to capture children with a physician diagnosis of c-TAK aged ≤ 18 years at disease onset. Data entry is hosted on Research Electronic Data Capture (REDCap) platform. Registry data includes demographic characteristics, clinical symptoms, organ involvement, laboratory features, vascular imaging, treatment, physician global assessment (PGA) of disease activity, and the features needed to calculate the Pediatric Vasculitis Activity Score (PVAS), Indian Takayasu Activity Score (ITAS2010 and ITAS-A), and 2018 European League Against Rheumatism (EULAR) criteria for active large vessel vasculitis. The assessment of damage using the pediatric vasculitis damage index (PVDI) is collected at follow-up. Data is collected retrospectively from patient medical records at diagnosis, 12-months, and at the final visit on file beyond 15 months (if applicable).
Results: As of abstract submission, the registry has collected data on 72 patients from 12 countries and 31 unique clinical sites. Additional enrollment has begun at 40 other sites, including from 13 additional counties. To date the cohort comprises 80% females. The average age at diagnosis is 12.14 +/- 4.81 years; the average time to diagnosis after first symptoms is 7.53 +/- 13.07 months; 66% received intravenous pulse glucocorticoids (GC) at time of diagnosis, while 85% were started on oral GC. Primary treatments (n=63) prescribed at time of diagnosis include methotrexate (62%), anti-TNF biologic (27%) cyclophosphamide (24%), ongoing intermittent pulse IV GC (11%), and tocilizumab (11%). Secondary treatments included antihypertensives (54%) and anti-coagulants (48%).
Conclusion: Creation of an international c-TAK registry is feasible owing to global collaborations between member sites of CARRA and PReS, and the capabilities for electronic communications and data transmission. Registry data will improve knowledge of presenting symptoms and treatment of c-TAK and increase understanding of the value of current disease activity measures. The successful realization of further project aims is expected.
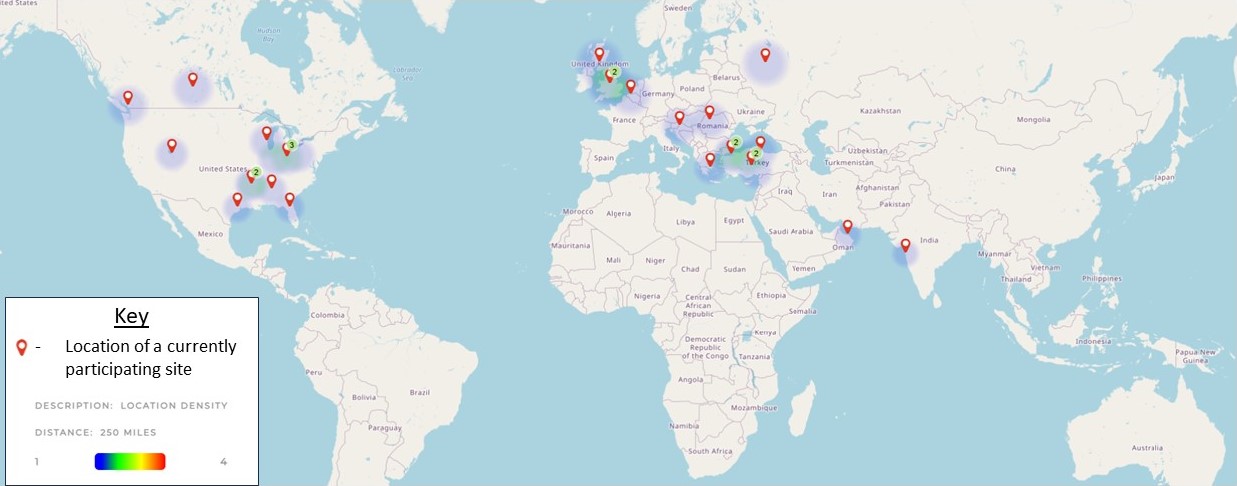 Location of clinical sites that have patients included in the current abstract submission
Location of clinical sites that have patients included in the current abstract submission
To cite this abstract in AMA style:
Kasap Cuceoglu M, Bistolarides J, Sestan M, Gagne S, McPhate N, Morishita K, Sivaraman V, Wagner-Weiner L, Özen S, Jelusic M, Cabral D. Preliminary Results of a Large, Global Registry Characterizing Childhood-Onset Takayasu Arteritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/preliminary-results-of-a-large-global-registry-characterizing-childhood-onset-takayasu-arteritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/preliminary-results-of-a-large-global-registry-characterizing-childhood-onset-takayasu-arteritis/
