Session Information
Date: Tuesday, October 28, 2025
Title: (1990–2014) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with gout are frequently treated in the emergency department (ED) for flares but may not receive consistent outpatient care. Approaches to mitigate this care pattern are understudied. We tested whether a direct-to-patient multimodal behavioral intervention could improve outpatient follow-up for gout after an ED visit.
Methods: We conducted a multicenter parallel group randomized clinical trial at 4 geographically diverse sites in the U.S. Participants were randomized 2:1 to the intervention or usual care (control) groups. The intervention included: 1) a storytelling video with gout patient testimonials, and 2) patient navigation whereby patients were contacted by phone after their ED visit by lay navigators to encourage outpatient follow up for gout. We assessed outcomes by phone surveys at 3-months. The primary outcome was the proportion of participants with an outpatient visit addressing gout in the 3-months post-ED visit, assessed by self-report and electronic health record (EHR) review. Secondary outcomes included rates of gout medication use, opioid use, and acute care utilization in the 3 months post-ED visit (documented in EHR, and as binary composite outcome including self-report). We performed intent-to-treat (ITT) and per-protocol (PP) analyses (i.e., intervention participants that viewed the storytelling video and 1+ navigator interaction). Differences in proportions were compared using Chi square tests and means using t-tests.
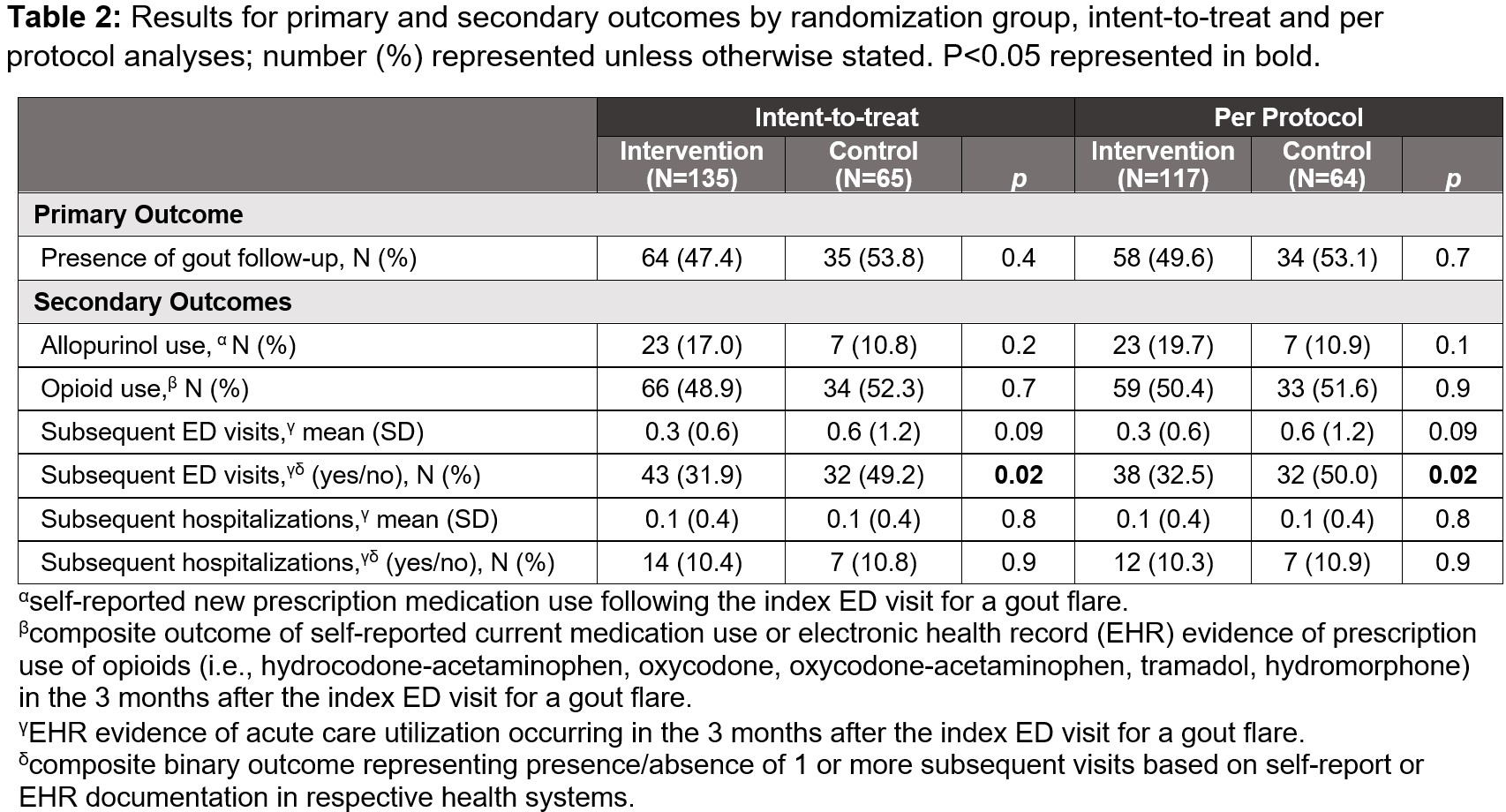
Results: From June 2021 to June 2024, 200 individuals (mean age 58 years; 76% men, 69% Black), were enrolled, with 135 randomized to intervention and 65 to control groups. Baseline characteristics are reported in Table 1. In ITT analyses, we found no significant differences between intervention vs control groups in rates of outpatient gout follow-up (47% vs 54%, p=0.4) (Table 2). Of gout follow-up visits captured in the EHR, a majority occurred with primary care (60%) followed by internal medicine subspecialties (29%). In the intervention group compared to control, there were no statistically significant differences in self-reported new allopurinol use post-ED (17% vs 11%, p=0.2). Among those who reported past or present allopurinol use, new initiation post-ED visit was more common in the intervention group (23/55 [42%] vs 7/32 [22%], p=0.05). Intervention group participants were less likely to have subsequent ED visits (intervention vs control 32% vs 49%, p=0.02) and there was a non-significant trend for fewer subsequent ED visits documented in the EHR at each site (intervention vs control 0.3 vs 0.6 visits, p=0.09). There were no differences in opioid prescription use or incident hospitalizations between groups. In PP analyses, trends were similar to ITT analysis with no significant differences between groups.
Conclusion: While our intervention did not lead to a significant improvement in outpatient gout follow-up, we did observe trends in improved uptake of allopurinol initiation and reduction in repeat ED visits. The findings suggest that while patient activation strategies may play a role, achieving improvements in routine gout follow-up care likely requires multifaceted approaches beyond behavioral interventions targeted to patients alone.
To cite this abstract in AMA style:
Jackson L, Begum R, Cutter G, Choi H, Faine B, Singer N, McCormick N, shokoohi h, Parry B, Nelson O, Booth J, Armor Z, Osborne J, Saag K, Danila M. Storytelling and Navigation to Improve Gout Follow-up: A Multicenter, Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/storytelling-and-navigation-to-improve-gout-follow-up-a-multicenter-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/storytelling-and-navigation-to-improve-gout-follow-up-a-multicenter-randomized-controlled-trial/

.jpg)