Session Information
Session Type: Abstract Session
Session Time: 11:15AM-11:30AM
Background/Purpose: Results from the ongoing open-label extension (OLE) of the MANDARA trial (NCT04157348) have demonstrated the efficacy of two years of anti-interleukin-5/receptor (anti-IL-5/R) therapies in inducing and maintaining remission in more than half of patients with eosinophilic granulomatosis with polyangiitis (EGPA). This analysis describes the types of relapses occurring in patients during the combined double-blind (DB) period and first year of the OLE.
Methods: 140 patients were randomized to receive either benralizumab (n=70) or mepolizumab (n=70) in the DB period of the MANDARA trial. After completing the 52-week DB period, 128 patients entered an OLE where they continued benralizumab (benra/benra; n=66) or switched from mepolizumab to benralizumab (mepo/benra; n=62) along with oral glucocorticoid (OGC) therapy, which could be tapered based on disease status. Relapse was defined as the presence of 1) active vasculitis (Birmingham Vasculitis Activity Score [BVAS] >0); OR asthma; OR sinonasal disease, leading to 2) an increase in OGC dose to >4 mg/day; OR initiation or increase in other immunosuppressive therapy; OR hospitalization for worsening EGPA. In this analysis, relapses were classified as asthma-related, sinonasal-related, ‘other’ (i.e., not asthma or sinonasal-related), or a combination of these three types for a total of seven types of relapses.
Results: During the DB period, 21 (30%) in each treatment group experienced a relapse. Among the 128 patients who entered the OLE, 15 (22.7%) patients in the benra/benra group and 20 (32.3%) patients in the mepo/benra group experienced a relapse. During the study, there were 127 relapse events reported during the DB (n=64) and first year of the OLE (n=63). Of these, 60 relapses occurred in the benralizumab (during the DB) or benra/benra group, and 67 in the mepolizumab (during the DB) or mepo/benra group (Figure 1). Over the two-year period, 103 (81.1%) of relapses involved asthma and/or sinonasal disease: 40 (31.5%) asthma, 27 (21.3%) sinonasal, 16 (12.6%) asthma-sinonasal, and 8 (6.3%) asthma-sinonasal-other. There were 24 (18.9%) exclusively ‘other’ types of relapses. The most common disease manifestations associated with the ‘other’ category of relapse were arthralgia/arthritis, myalgia, and sensory peripheral neuropathy (Figure 2).
Conclusion: Among patients with EGPA treated with anti-IL-5/R therapies, the majority of relapses are airway-related, including asthma and/or sinonasal relapses. Arthralgia/arthritis, myalgia, and sensory peripheral neuropathy are the most common manifestations of non-airway relapses.
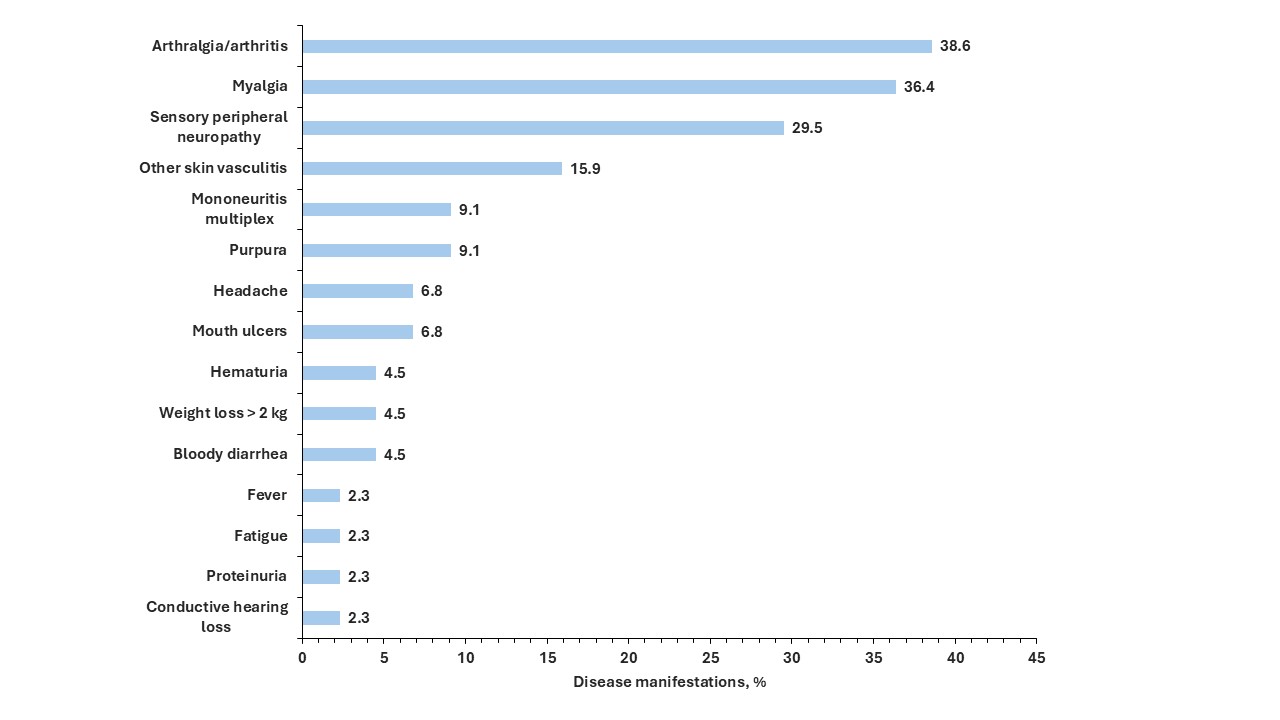 Figure 1. Subtypes of relapses in patients with eosinophilic granulomatosis with polyangiitis treated with anti-IL-5/R therapies
Figure 1. Subtypes of relapses in patients with eosinophilic granulomatosis with polyangiitis treated with anti-IL-5/R therapies
.jpg) Figure 2. Disease manifestations of relapses of eosinophilic granulomatosis with polyangiitis involving non-airway manifestations among patients treated with anti-IL-5/R therapies during the one-year double-blind period and the first year of the open-label extension periods in MANDARA. Relapses may be associated with more than one disease manifestation.
Figure 2. Disease manifestations of relapses of eosinophilic granulomatosis with polyangiitis involving non-airway manifestations among patients treated with anti-IL-5/R therapies during the one-year double-blind period and the first year of the open-label extension periods in MANDARA. Relapses may be associated with more than one disease manifestation.
To cite this abstract in AMA style:
Pagnoux C, Bourdin A, Hellmich B, Khalidi N, Jackson D, Jayne D, Nair P, Specks U, Terrier B, Börjesson Sjö L, Jain P, Lal A, Necander S, Walton C, Wechsler M, Merkel P. Classification of Relapses of Eosinophilic Granulomatosis with Polyangiitis After Two-Years of Treatment with Anti-Interleukin-5/Receptor Therapy [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/classification-of-relapses-of-eosinophilic-granulomatosis-with-polyangiitis-after-two-years-of-treatment-with-anti-interleukin-5-receptor-therapy/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/classification-of-relapses-of-eosinophilic-granulomatosis-with-polyangiitis-after-two-years-of-treatment-with-anti-interleukin-5-receptor-therapy/
