Session Information
Session Type: Abstract Session
Session Time: 10:00AM-10:15AM
Background/Purpose: Evaluating the effectiveness and safety profile of treatment strategies for the induction of remission of individuals with newly diagnosed AAV is important for guiding care. We expanded AAV-Sim, a previously developed and validated microsimulation model of ANCA-Associated Vasculitis (AAV). Thus far, the model has simulated strategies to maintain remission in AAV. We expanded the model to project clinical outcomes in the setting of induction of remission in individuals with newly-diagnosed AAV receiving rituximab along with a reduced-dose glucocorticoid (GC) taper.
Methods: We expanded AAV-Sim, a C++-based microsimulation model with a monthly timestep that simulates clinical events among individuals with AAV undergoing treatment, to incorporate outcomes during induction of remission among those with newly-diagnosed disease (Figure). At model start, all simulated individuals are newly-diagnosed with active disease and are at risk of severe infection, end-stage renal disease (ESRD), diabetes mellitus, and death. The model assumes all individuals receive rituximab as remission induction therapy. Remission within 6 months is based on a probability of entering remission during each month. Once an individual enters remission, there is a monthly probability of minor or major relapse. Inputs were derived from outcomes observed in clinical trials (RAVE or LoVAS, depending on outcome of interest) (Table 1) or from an observational cohort of individuals with AAV seen in a large academic center. We also included multipliers for certain parameters (e.g., risk of ESRD among those with vs. without baseline renal involvement from AAV) from observational data. We performed validation by using the mean average percent error (MAPE), a measure of the accuracy of predictions in forecasting methods, to compare the predicted outcomes in AAV-Sim with observed outcomes. MAPE results less than 10% are considered to be highly accurate and 11-20% are considered very good. We projected model outcomes over 6 months for: time to remission, minor and major relapse, severe infection, incident diabetes mellitus, ESRD, and death.
Results: Using the expanded AAV-Sim model, we projected outcomes similar to observed outcomes in clinical trials and observational data, including: time to remission (2.44 months in AAV-Sim vs. mean of 2.37 months in RAVE, MAPE 3.0%), minor relapse (2.39% in AAV-Sim vs. 2.90% in LoVAS, MAPE 17.6%), major relapse (1.32% in AAV-Sim vs. 1.45% in LoVAS, MAPE 9.0%), ≥1 severe infection (6.66% in AAV-Sim vs. 7.24% in LoVAS, MAPE 8.0%), incident diabetes (11.54% in AAV-Sim vs. 13.04% in LoVAS, MAPE 11.5%), incident ESRD (0.40% in AAV-Sim vs. 0.51% in MGB AAV cohort, MAPE 21.6%), and death (2.71% in AAV-Sim vs. 2.90% in LoVAS, MAPE 6.6%) (Table 2).
Conclusion: We expanded and validated a model to simulate induction of remission in AAV. The expanded AAV-Sim model can be used to project clinical outcomes after induction of remission. These results can be used and further expanded upon to address important clinical questions in AAV in research studies and in clinical trial design. Further expansion of the model will evaluate outcomes between those receiving different GC regimens.
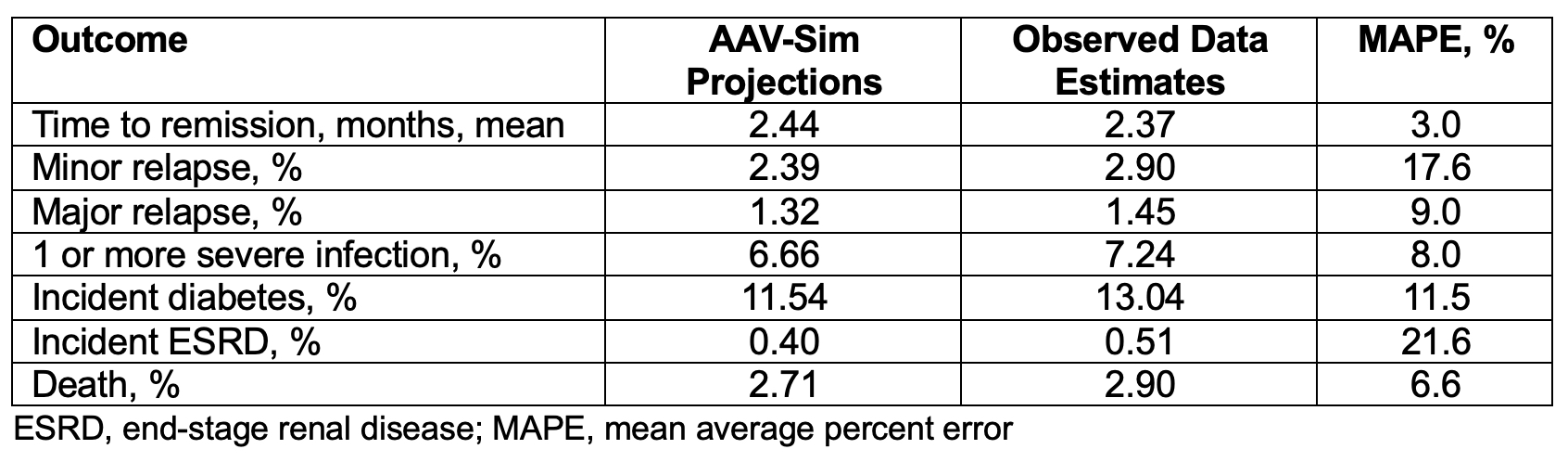 Figure. Schematic of Health States and Transitions in the Expanded AAV-Sim Model that Incorporates Induction of Remission in People with Newly-Diagnosed Active ANCA-Associated Vasculitis
Figure. Schematic of Health States and Transitions in the Expanded AAV-Sim Model that Incorporates Induction of Remission in People with Newly-Diagnosed Active ANCA-Associated Vasculitis
.jpg) Table 1. Selected Input Parameters for the Expanded AAV-Sim Model that Includes Induction of Remission in People with Newly-Diagnosed ANCA-Associated Vasculitis
Table 1. Selected Input Parameters for the Expanded AAV-Sim Model that Includes Induction of Remission in People with Newly-Diagnosed ANCA-Associated Vasculitis
.jpg) Table 2. Model-Projected Outcomes versus Observed Outcomes over 6 months during Induction of Remission in Newly Diagnosed Disease Using the Expanded AAV-Sim Model
Table 2. Model-Projected Outcomes versus Observed Outcomes over 6 months during Induction of Remission in Newly Diagnosed Disease Using the Expanded AAV-Sim Model
To cite this abstract in AMA style:
Patel N, Wu A, Miloslavsky E, Merkel P, Stone J, Choi H, Wallace Z, Hyle E. Development and Validation of a Simulation Model for Induction of Remission in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/development-and-validation-of-a-simulation-model-for-induction-of-remission-in-antineutrophil-cytoplasmic-antibody-associated-vasculitis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-validation-of-a-simulation-model-for-induction-of-remission-in-antineutrophil-cytoplasmic-antibody-associated-vasculitis/
