Session Information
Session Type: Abstract Session
Session Time: 10:45AM-11:00AM
Background/Purpose: RA-associated interstitial lung disease (RA-ILD) causes substantial morbidity and mortality in RA. Despite this, a limited number of clinical and genetic risk factors have been identified. Recently, a combined clinical-genetic risk score (VARA-ILD Combined Risk Score) has been shown to enhance identification of ILD among RA patients (Wheeler et al., Rheumatology 2024). We performed external validation of that combined risk score in a cohort of early RA patients from northern Sweden.
Methods: Participants were enrolled in an inception RA cohort from northern Sweden who were diagnosed with RA between 1996-2016. Clinical data including age, sex, smoking history, and disease activity score with 28-joint count (DAS28) were collected routinely by treating rheumatologists. RF and anti-CCP were assessed at baseline using standard lab methods. ILD screening was performed per clinical indication, with ILD diagnosis based on consistent chest x-ray and/or high-resolution chest CT findings. Genotyping was performed on DNA collected at baseline using the Illumina Global Screening Array, and single nucleotide polymorphisms (SNPs) included in the VARA-ILD Genetic Risk Score (GRS) were extracted. The VARA-ILD Combined Risk score was calculated for each participant and evaluated via multivariable regression. Overall model performance was assessed using the area under the receiver operating characteristic curve (AUC). Sensitivity and specificity were calculated at the recommended cut-point value (0.05) and at the cut-point that optimized Youden’s index in this cohort.
Results: We studied 1,118 RA participants (70% female), with ILD present in 60 (5.4% prevalence). RA-ILD participants were older, had more RF positivity, and had higher disease activity (Table 1). Among SNPs included in the VARA-ILD GRS, MUC5B rs35705950 (OR 3.70, 95% CI 2.18-6.32) and FAM13A rs2609255 (OR 1.91, 95% CI 1.13-3.25) were individually associated with RA-ILD (Table 2). Within the VARA-ILD combined risk score regression model, the largest effects were seen with the VARA-ILD GRS (OR 2.36, 95% CI 1.24-4.48) and RF positivity (OR 2.96, 95% CI 1.14-7.70). The VARA-ILD Combined Risk Score enhanced discrimination of ILD (AUC 0.75, 95% CI 0.69-0.81) over the GRS alone (AUC 0.62, 95% CI 0.54-0.69; Figure 1). At the recommended cut-point of 0.05, the VARA-ILD Combined Risk Score yielded 93% sensitivity and would eliminate 36% of the cohort from undergoing additional testing for ILD. We also calculated an optimal cut-point of 0.08 within this cohort which would yield a sensitivity of 79% and specificity of 95%.
Conclusion: The VARA-ILD GRS and VARA-ILD Combined Risk Score were externally validated in a cohort of early RA patients in northern Sweden. Given the differences in study population between this cohort and the VARA-ILD derivation cohort, our findings demonstrate the generalizability and potential clinical utility of these risk stratification tools for RA-ILD.
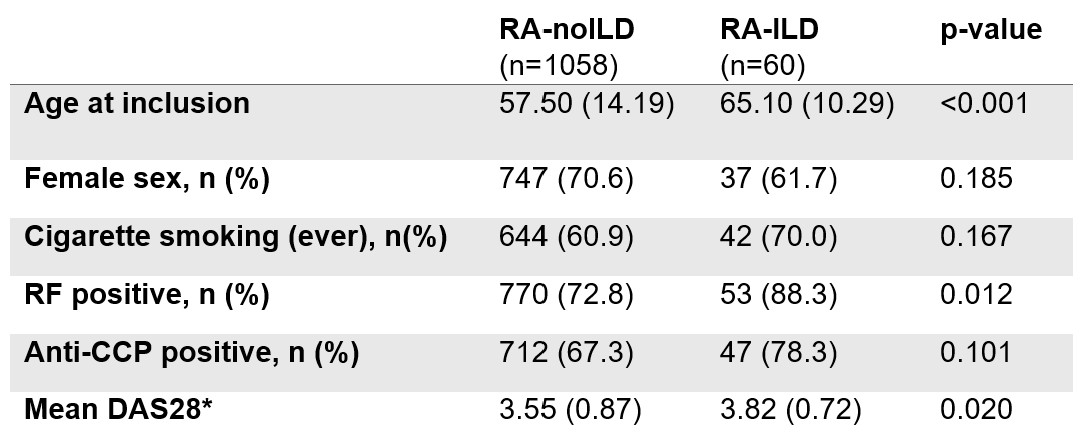 Table 1. Characteristics of participants in the northern Sweden early RA cohort.
Table 1. Characteristics of participants in the northern Sweden early RA cohort.
Values shown as mean (standard deviation) unless specified.
*DAS28 averaged from prior 24 months.
Abbreviations: rheumatoid arthritis without interstitial lung disease (RA-noILD), rheumatoid arthritis-associated interstitial lung disease (RA-ILD), rheumatoid factor (RF), anti-citrullinated protein antibody (ACPA), disease activity score with 28 joint count (DAS28)
.jpg) Table 2. Genotype frequencies and OR of the effect allele for each single nucleotide polymorphism included in the VARA-ILD genetic risk score.
Table 2. Genotype frequencies and OR of the effect allele for each single nucleotide polymorphism included in the VARA-ILD genetic risk score.
Abbreviations: rheumatoid arthritis without interstitial lung disease (RA-noILD), rheumatoid arthritis-associated interstitial lung disease (RA-ILD), odds ratio (OR), confidence interval (CI)
.jpg) Figure 1. Comparison of receiver operating characteristic curves for the VARA-ILD Combined Risk Score and GRS alone.
Figure 1. Comparison of receiver operating characteristic curves for the VARA-ILD Combined Risk Score and GRS alone.
Abbreviations: area under the receiver operating characteristic curve (AUC)
To cite this abstract in AMA style:
Brink M, Wheeler A, England B, Rantapaa-Dahlqvist S. External validation of a combined clinical and genetic risk score for the identification of interstitial lung disease in rheumatoid arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/external-validation-of-a-combined-clinical-and-genetic-risk-score-for-the-identification-of-interstitial-lung-disease-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/external-validation-of-a-combined-clinical-and-genetic-risk-score-for-the-identification-of-interstitial-lung-disease-in-rheumatoid-arthritis/
