Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Immunological Complications of Medical Therapy (1728–1733)
Session Type: Abstract Session
Session Time: 10:45AM-11:00AM
Background/Purpose: Chimeric Antigen Receptor (CAR-T) therapy has transformed the management of hematologic cancers, with multiple products approved by the U.S. Food and Drug Administration (FDA). While immune-mediated toxicities such as cytokine release syndrome and neurotoxicity are well described, the spectrum of autoimmune adverse events (AEs) remain poorly defined. Emerging case reports and small series suggest a potential link between CAR-T and new-onset autoimmune conditions. As the use of CAR-T expands to rheumatologic conditions, understanding the autoimmune safety profile is critical.
Methods: We conducted a pharmacovigilance analysis of autoimmune AEs reported to the FDA Adverse Event Reporting System (FAERS) for each FDA-approved CAR-T product through March 2025. All individual case safety reports listing any of the CAR-T therapies—tisagenlecleucel (tisacel), axicabtagene ciloleucel (axicel), lisocabtagene maraleucel (lisocel), brexucabtagene autoleucel (brexucel), idecabtagene vicleucel (idecel), and ciltacabtagene autoleucel (ciltacel)—as the suspected drug were included. Autoimmune AEs were identified using standardized MedDRA terms. The patient characteristics including malignancy, CAR-T products and AEs were summarized. Disproportionality analysis was performed using Reported Odds Ratio (ROR).
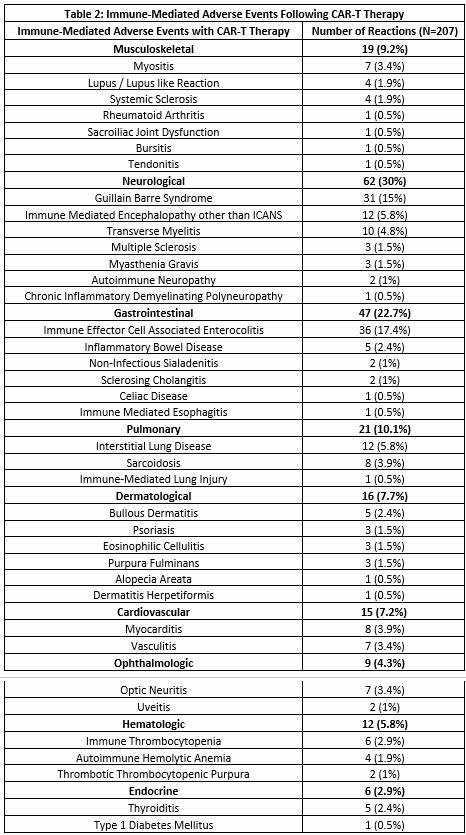
Results: There were 207 immune-mediated AEs with CAR-T reported in the FAERS database (Table 1 and 2). Compared to other products, Idecel was associated with development of sarcoidosis (ROR 17.15, CI 4.28-68.67, p< 0.0001). Ciltacel was associated with Guillain-Barre syndrome (ROR 7.31, CI 3.57-14.93, p< 0.0001) and immune effector cell associated enterocolitis (IEC-EC) (ROR 58.42, CI 17.90-190.62, p< 0.0001). Axicel was associated with dermal autoimmune AEs (Table 2) (ROR 3.65, CI 1.18-11.33, p=0.024). Tisacel was associated with myocarditis (ROR 6.20, CI 1.48-25.95, p=0.012) and vasculitis (ROR 4.96, CI 1.11-22.16, p=0.036). Compared to other malignancies with CAR-T approval, indolent B-cell lymphoma was associated with myocarditis (ROR 10.72, CI 2.07-55.40, p=0.004) and musculoskeletal autoimmune AEs (Table 2) (ROR 8.05, CI 2.21-29.37, p=0.001) - in particular, myositis (ROR 10.72, CI 2.07-55.40, p=0.004) and lupus/lupus-like reaction (ROR 13.37, CI 1.21-147.72, p=0.034). Follicular lymphoma in particular was associated with vasculitis (ROR 9.66, CI 1.002-93.06, p=0.049). Higher odd of developing optic neuritis was seen in patients age < 18 years (ROR 7.41, CI 1.35-40.55, p=0.02), acute B-cell lymphoblastic leukemia (ROR 21.78, CI 4.22-112.37, p=0.0002), and Tisacel (ROR 22.33, CI 2.69-185.53, p=0.004). Female sex and age >65 were more likely to develop IEC-EC (ROR 1.90, CI 0.90-4.0, p=0.089; ROR 2.04, CI 1.01-4.14, p=0.047).
Conclusion: This post-marketing pharmacovigilance analysis highlights product- and disease-specific patterns of autoimmune AEs following CAR-T therapy. While limited by FAERS reporting bias, these findings support the need for monitoring strategies and emphasize the importance of prospective studies to validate these associations. Integrating pharmacogenomic and immunologic correlates may further elucidate underlying mechanisms.
 Characteristics of Patients with Immune-Mediated Adverse Reactions Reported to the FDA Adverse Event Reporting System (FAERS)
Characteristics of Patients with Immune-Mediated Adverse Reactions Reported to the FDA Adverse Event Reporting System (FAERS)
.jpg) Immune-Mediated Adverse Events Following CAR-T Therapy
Immune-Mediated Adverse Events Following CAR-T Therapy
To cite this abstract in AMA style:
Gupta M, Graham C, Gupta S. A Post-Marketing Analysis of Autoimmune Toxicities Following Chimeric Antigen Receptor T-Cell Therapy Using the FDA Adverse Event Reporting System [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-post-marketing-analysis-of-autoimmune-toxicities-following-chimeric-antigen-receptor-t-cell-therapy-using-the-fda-adverse-event-reporting-system/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-post-marketing-analysis-of-autoimmune-toxicities-following-chimeric-antigen-receptor-t-cell-therapy-using-the-fda-adverse-event-reporting-system/
