Session Information
Date: Tuesday, October 28, 2025
Title: Plenary III (1722–1727)
Session Type: Plenary Session
Session Time: 9:15AM-9:30AM
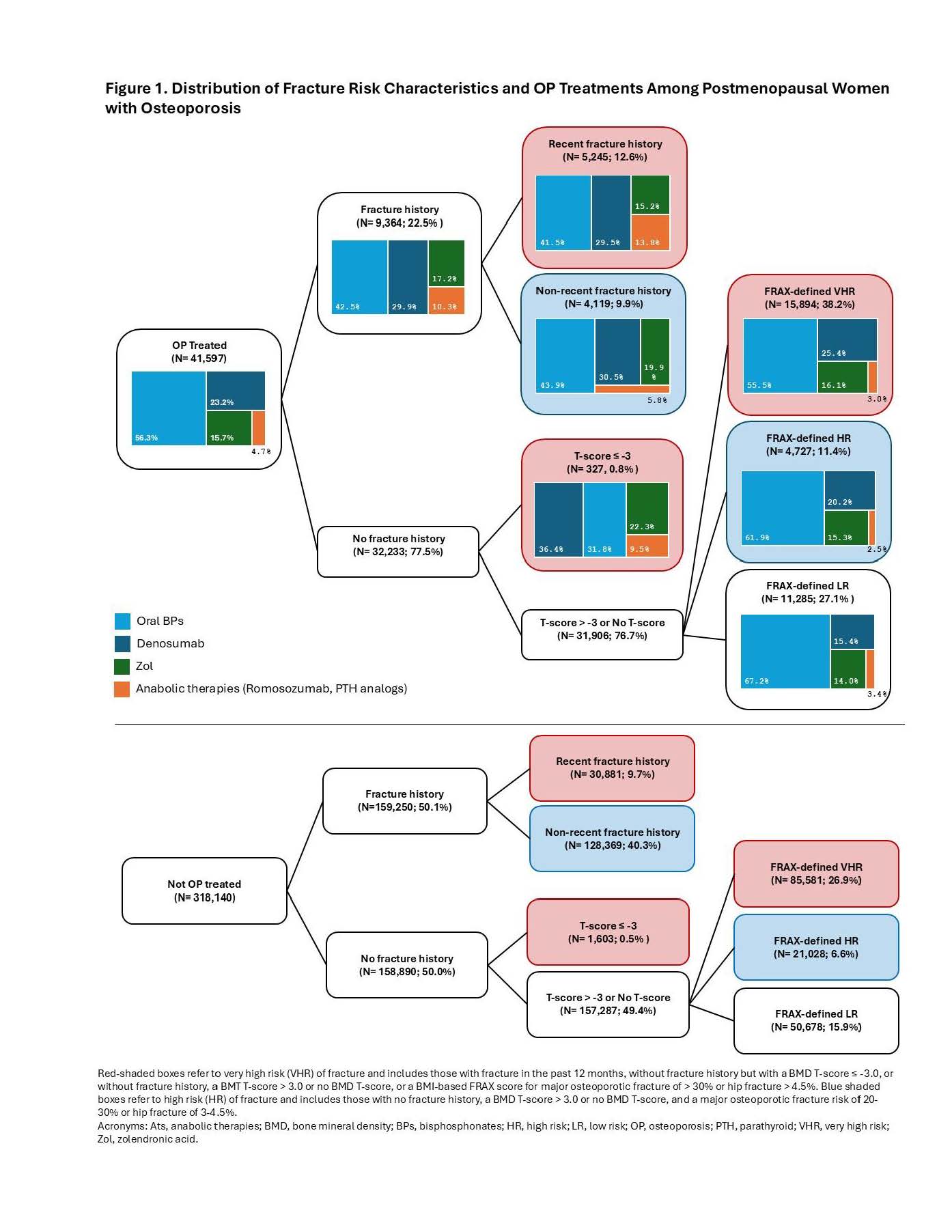
Background/Purpose: Current guidelines recommend treating women with postmenopausal osteoporosis (PMO) based on fracture risk. Anabolic therapy (AT), the RANKL inhibitor denosumab (Dmab), and intravenous zoledronic acid (ZOL) are recommended as initial therapy for patients at very high risk (VHR) for fracture. This study described the proportion of PMO women at VHR or high risk (HR) of fracture by treatment status in a commercially insured population in the US.
Methods: This retrospective cohort study used Optum® Market Clarity Bone Data. Women ≥55 years with a diagnosis of osteoporosis (OP), fracture, or OP medication use, ≥455 days of continuous enrollment, a valid BMI and race, and no history of Paget’s disease or metastatic cancer were included. Women who newly initiated an OP treatment from April 1, 2016 – September 30, 2023 were included in the treated cohort; date of treatment initiation was the index date. Women with no history of OP treatment in the 15-months prior to January 1, 2023 (index date) were included in the untreated cohort. Cohorts were not mutually exclusive. OP medications included oral bisphosphonates (BPs; alendronate, risedronate, ibandronate), Dmab, ZOL, and AT (romosozumab, parathyroid hormone analogues [teriparatide, abaloparatide]). HR and VHR were defined based on AACE guidelines for the treatment of PMO women and assessed over 15-months before index.
Results: Of the 41,597 treated PMO women identified (mean age=73 y), 51.6% met the VHR criteria, including 5,245 women with recent (≤12 months) fracture (12.6%), 327 with a BMD T-score ≤ -3.0 (0.8%), and 15,894 with a FRAX score indicating VHR (38.2%). Approximately 21.3% were HR; 27.1%, low risk (LR). The most frequent OP treatments overall and among fracture risk subgroups were oral BPs (56.3%) followed by Dmab (23.2%) and ZOL (15.7%). Only 4.7% of treated PMO women overall and 5.7% of treated VHR PMO women were using AT. Among the 318,140 untreated PMO women identified (mean age=74 y), 118,065 (37.1%) met the criteria for VHR; 149,397 (47.0%), HR; 50,678 (15.9%), LR.
Conclusion: More than 1/3 of untreated PMO women are at VHR for fracture and eligible for ATs, Dmab, or ZOL in accordance with treatment guidelines. Among treated VHR PMO women, over half were treated with oral BP in contradiction with current treatment guidelines. Only a small percentage were treated with ATs, which clinical studies have shown are the most effective initial therapy for PMO women at VHR for fracture.
To cite this abstract in AMA style:
Chien H, Kim M, McDermott M, Oates M, Li X, Yoon L. Distribution of Fracture Risk Status and Osteoporosis Treatment Use Among Postmenopausal Women with Osteoporosis in the United States [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/distribution-of-fracture-risk-status-and-osteoporosis-treatment-use-among-postmenopausal-women-with-osteoporosis-in-the-united-states/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/distribution-of-fracture-risk-status-and-osteoporosis-treatment-use-among-postmenopausal-women-with-osteoporosis-in-the-united-states/