Session Information
Date: Monday, October 27, 2025
Title: Abstracts: Miscellaneous Rheumatic & Inflammatory Diseases II: Models and Mechanisms (1662–1667)
Session Type: Abstract Session
Session Time: 1:15PM-1:30PM
Background/Purpose: Acute exacerbation (AE) of rheumatic disease-related interstitial lung disease (RD-ILD) is a severe and potentially life-threatening condition, while there remains no clear consensus of evidence-based therapy guidelines. Conventional treatments rely on high-dose corticosteroids and broad immunosuppressants, which carry significant toxicity and variable efficacy. This study aims to evaluate the effectiveness and safety of the interleukin-6 (IL-6) receptor inhibitor tocilizumab in managing AE-RD-ILD.
Methods: This prospective observational study enrolled hospitalized AE-RD-ILD patients treated with tocilizumab between January 2023 and May 2024. Retrospective controls were selected from the same institution and period, receiving corticosteroids with or without conventional immunosuppressants (e.g., cyclophosphamide, mycophenolate mofetil). All patients met diagnostic criteria for AE-RD-ILD, including: acute worsening of respiratory distress requiring additional oxygen supply within 1-month, High-resolution computed tomography (HRCT) demonstrating new bilateral ground-glass opacities and/or consolidations superimposed on pre-existing ILD patterns, and confirmed underlying rheumatic disease by clinical and serological criteria. Exclusion criteria included active infections, severe comorbidities (e.g., heart failure), or recent use of interleukin-6 inhibitors. Primary outcome was complete response (CR) at 24 weeks, defined by resolution of dyspnea, radiographic improvement, steroid reduction (≤15 mg/day prednisone equivalent), and survival without severe adverse events or lung transplantation.
Results: A total of 45 patients were included in the analysis (tocilizumab group: n=26; control group: n=19). Baseline characteristics were comparable with no significant differences in median age (62 vs. 63 years, P=0.918) or ILD duration (16 vs. 15 months, P=0.991). Among enrolled patients, 39 (86.6%) met American College of Rheumatology (ACR) classification criteria for defined rheumatic diseases (dermatomyositis: 33, rheumatoid arthritis: 3, systemic sclerosis: 2, ANCA associated vasculitis: 1), while the remaining 6 (13.3%) were diagnosed as undifferentiated connective tissue disease. The tocilizumab group achieved a significantly higher 24-week CR rate of 80.8% (21/26) compared to 10.5% (2/19) in the control group (P< 0.001), with 26% lower cumulative prednisone use (3955 vs. 5375 mg, P=0.017), reduced maintenance doses by 50% (10 vs. 20 mg/day, P< 0.001), fewer new acute exacerbations (3 vs. 8 events, P=0.045), and higher oxygen discontinuation rates (76.9% vs. 5.3%, P< 0.001). Safety outcomes favored tocilizumab, with significantly fewer infections (7 vs. 21 events, P< 0.001) and lower composite mortality/lung transplantation rates (3.8% vs. 31.6%, P=0.034). Subgroup analyses showed consistent benefits across demographics (P for interaction >0.05).
Conclusion: AE-RD-ILD patients treated with the tocilizumab demonstrated higher CR rate at 24 weeks, reduced steroid requirement, with a favorable safety profile, which support the potential for tocilizumab to addresses the critical unmet need in managing AE-RD-ILD.
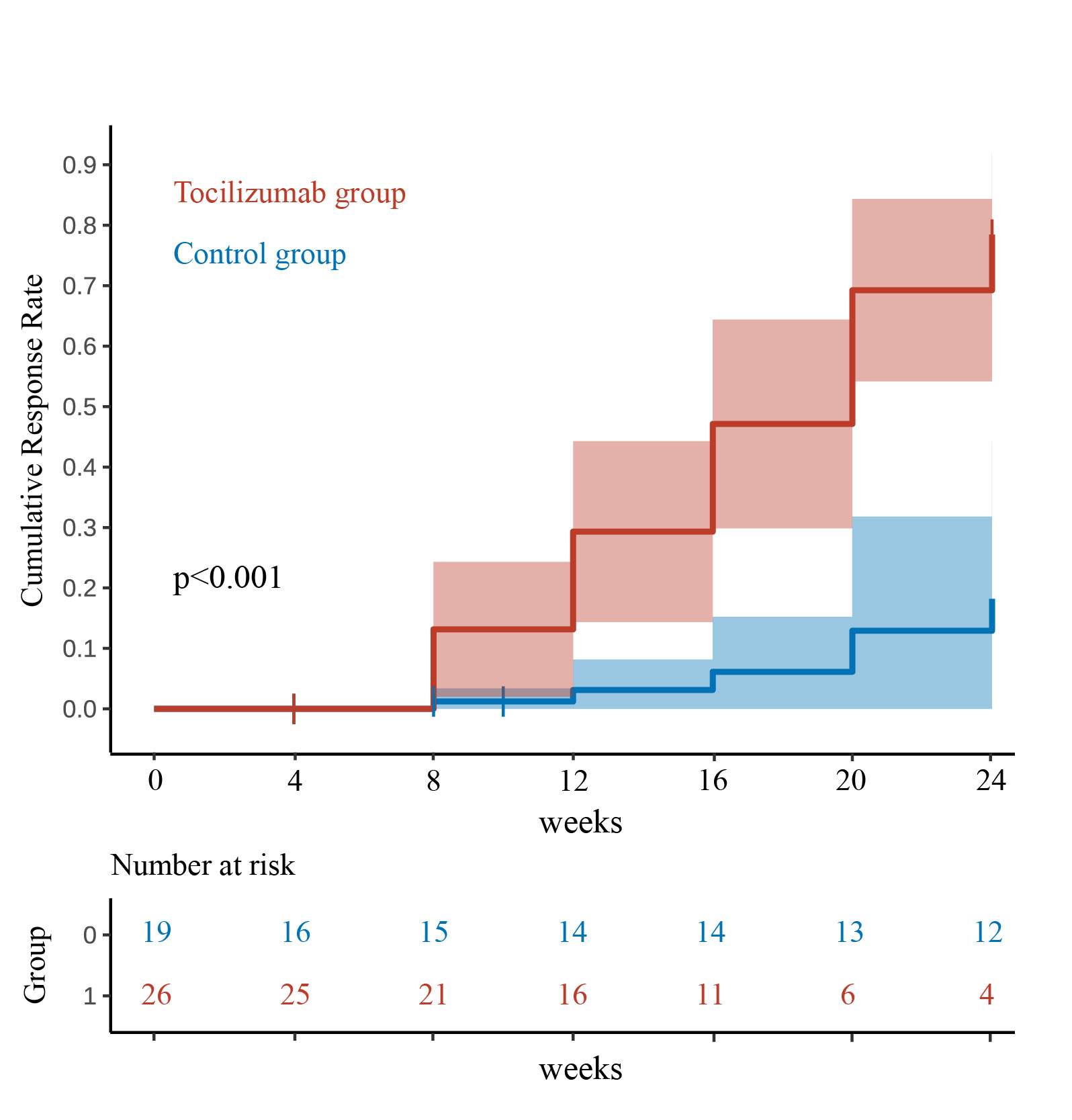 Figure 1. Cumulative response curve for the tocilizumab and control groups within 24 weeks.
Figure 1. Cumulative response curve for the tocilizumab and control groups within 24 weeks.
Direct Standardization adjusted by variables of sex, age, course, PaO2/FiO2, forced vital capacity predicted, baseline immunosuppressive therapy, the maximum daily dose of steroids after AE, anti-fibrotic drugs, B cells, and ILD field. One death and five deaths were censored in the tocilizumab group and control group respectively.
The complete response rate was 80.8% (21/26) compared to 10.5% (2/19) (p < 0.001). The difference between the response rates for two groups after 8, 12, 16, 20 and 24 weeks were 11.9% (95% CI 0.6-23.3), 26.2% (10.4-42.0), 41.0% (21.5-60.5), 56.3% (32.2-80.5) and 60.3% (31.1-89.5), all P < 0.05.
.jpg) Figure 2. Respiratory Support Parameters & CT Images: Pre-Post Treatment Comparison.
Figure 2. Respiratory Support Parameters & CT Images: Pre-Post Treatment Comparison.
B: A 45-year-old male with anti-Jo-1 syndrome ILD. Baseline HRCT showed non-specific interstitial pneumonia (NSIP) pattern with homogeneous ground-glass opacities and spared subpleural parenchyma. After 6 months, acute exacerbation led to respiratory failure with increased ground-glass opacities and diffuse lung involvement without volume loss. After high-dose prednisone and tocilizumab treatment, significant absorption and improvement of pulmonary lesions were observed. C: A 41-year-old female with anti-synthetase syndrome ILD. Baseline HRCT showed NSIP pattern. After 5 months, acute respiratory failure occurred with diffuse ground-glass opacities, consolidation, increased subpleural reticulation, and traction bronchiectasis severity, with volume loss. One-month post-treatment, pulmonary lesions improved.
.jpg) Figure 3. Comparison of baseline and post-treatment laboratory parameters between Tocilizumab responders (n=21) and non-responders (n=4).
Figure 3. Comparison of baseline and post-treatment laboratory parameters between Tocilizumab responders (n=21) and non-responders (n=4).
Heatmaps were used to display data before and after treatment in tocilizumab group, with each variable standardized; standardized values >2 was displayed as 2, and those less than -2 as -2, with statistically significant between-group differences (P < 0.05) indicated by an asterisk (*).
Mono: monocytes; LY: lymphocytes; NK: natural killer; Th: T helper cells; Ts: T suppressor cells; ESR: erythrocyte sedimentation rate; IgG: immunoglobulin G; IL: interleukin; LDH: lactate dehydrogenase; NLR: neutrophil-to-lymphocyte ratio; KL6: Krebs von den Lungen-6; CRP: C-reactive protein; CK: creatine kinase.
To cite this abstract in AMA style:
Xu W, Ma Y, Ye S, Fu Q. Tocilizumab for acute exacerbation of rheumatic disease-related interstitial lung disease: A prospective real-world study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/tocilizumab-for-acute-exacerbation-of-rheumatic-disease-related-interstitial-lung-disease-a-prospective-real-world-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-for-acute-exacerbation-of-rheumatic-disease-related-interstitial-lung-disease-a-prospective-real-world-study/
